Abstract
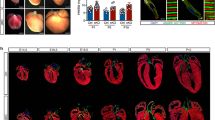
Epigenetic changes on DNA and chromatin are implicated in cell differentiation and organogenesis. For the heart, distinct histone methylation profiles were recently linked to stage-specific gene expression programs during cardiac differentiation in vitro. However, the enzymes catalyzing these modifications and the genes regulated by them remain poorly defined. We therefore decided to identify the epigenetic enzymes that are potentially involved in cardiomyogenesis by analyzing the expression profile of the 85 genes encoding the epigenetic-related proteins in mouse cardiomyocytes (CMs), and then study how they affect gene expression during differentiation and maturation of this cell type. We show here with gene expression screening of epigenetic enzymes that the highly expressed H3 methyltransferase disruptor of telomeric silencing 1-like (DOT1L) drives a transitional pattern of di-methylation on H3 lysine 79 (H3K79) in CMs at different stages of differentiation in vitro and in vivo. Through a genome-wide chromatin-immunoprecipitation DNA-sequencing approach, we found H3K79me2 enriched at genes expressed during cardiac differentiation. Moreover, knockdown of Dot1L affected the expression of H3K79me2-enriched genes. Our results demonstrate that histone methylation, and in particular DOT1L-mediated H3K79me2 modification, drives cardiomyogenesis through the definition of a specific transcriptional landscape.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
Abbreviations
- ChIP-seq:
-
chromatin immunoprecipitation-sequencing
- CM:
-
cardiomyocyte
- CMa:
-
adult cardiomyocyte
- CMp:
-
pup cardiomyocyte
- DNMT:
-
DNA methyltranferase
- DOT1L:
-
telomeric silencing-1-like H3K79 methyltransferase
- GEO:
-
Gene Expression Omnibus
- H:
-
histone
- HAT:
-
histone acetyltransferase
- HDAC:
-
histone deacetylase
- HDM:
-
histone demethylase
- HMT:
-
histone methyltransferase
- me:
-
methylation
- mES:
-
mouse embryonic stem cell
- TSS:
-
transcription start site
References
Evans SM, Yelon D, Conlon FL, Kirby ML . Myocardial lineage development. Circ Res 2010; 107: 1428–1444.
Garry DJ, Olson EN . A common progenitor at the heart of development. Cell 2006; 127: 1101–1104.
Martin-Puig S, Wang Z, Chien KR . Lives of a heart cell: tracing the origins of cardiac progenitors. Cell Stem Cell 2008; 2: 320–331.
Bernstein BE, Mikkelsen TS, Xie X, Kamal M, Huebert DJ, Cuff J et al. A bivalent chromatin structure marks key developmental genes in embryonic stem cells. Cell 2006; 125: 315–326.
Guenther MG, Levine SS, Boyer LA, Jaenisch R, Young RA . A chromatin landmark and transcription initiation at most promoters in human cells. Cell 2007; 130: 77–88.
Meissner A . Epigenetic modifications in pluripotent and differentiated cells. Nat Biotechnol 2010; 28: 1079–1088.
Bruneau BG . The developmental genetics of congenital heart disease. Nature 2008; 451: 943–948.
Barski A, Cuddapah S, Cui K, Roh TY, Schones DE, Wang Z et al. High-resolution profiling of histone methylations in the human genome. Cell 2007; 129: 823–837.
Kouzarides T . Chromatin modifications and their function. Cell 2007; 128: 693–705.
Wamstad JA, Alexander JM, Truty RM, Shrikumar A, Li F, Eilertson KE et al. Dynamic and coordinated epigenetic regulation of developmental transitions in the cardiac lineage. Cell 2012; 151: 206–220.
Paige SL, Thomas S, Stoick-Cooper CL, Wang H, Maves L, Sandstrom R et al. A temporal chromatin signature in human embryonic stem cells identifies regulators of cardiac development. Cell 2012; 151: 221–232.
Ohtani K, Zhao C, Dobreva G, Manavski Y, Kluge B, Braun T et al. Jmjd3 controls mesodermal and cardiovascular differentiation of embryonic stem cells. Circ Res 2013; 113: 856–862.
Delgado-Olguin P, Huang Y, Li X, Christodoulou D, Seidman CE, Seidman JG et al. Epigenetic repression of cardiac progenitor gene expression by Ezh2 is required for postnatal cardiac homeostasis. Nat Genet 2012; 44: 343–347.
He A, Ma Q, Cao J, von Gise A, Zhou P, Xie H et al. Polycomb repressive complex 2 regulates normal development of the mouse heart. Circ Res 2012; 110: 406–415.
Papait R, Cattaneo P, Kunderfranco P, Greco C, Carullo P, Guffanti A et al. Genome-wide analysis of histone marks identifying an epigenetic signature of promoters and enhancers underlying cardiac hypertrophy. Proc Natl Acad Sci USA 2013; 110: 20164–20169.
Porrello ER, Mahmoud AI, Simpson E, Hill JA, Richardson JA, Olson EN et al. Transient regenerative potential of the neonatal mouse heart. Science 2011; 331: 1078–1080.
Marson A, Levine SS, Cole MF, Frampton GM, Brambrink T, Johnstone S et al. Connecting microRNA genes to the core transcriptional regulatory circuitry of embryonic stem cells. Cell 2008; 134: 521–533.
Mikkelsen TS, Ku M, Jaffe DB, Issac B, Lieberman E, Giannoukos G et al. Genome-wide maps of chromatin state in pluripotent and lineage-committed cells. Nature 2007; 448: 553–560.
Ross-Innes CS, Stark R, Teschendorff AE, Holmes KA, Ali HR, Dunning MJ et al. Differential oestrogen receptor binding is associated with clinical outcome in breast cancer. Nature 2012; 481: 389–393.
Jones B, Su H, Bhat A, Lei H, Bajko J, Hevi S et al. The histone H3K79 methyltransferase Dot1L is essential for mammalian development and heterochromatin structure. PLoS Genet 2008; 4: e1000190.
Nguyen AT, Xiao B, Neppl RL, Kallin EM, Li J, Chen T et al. DOT1L regulates dystrophin expression and is critical for cardiac function. Genes Dev 2011; 25: 263–274.
Feng Q, Wang H, Ng HH, Erdjument-Bromage H, Tempst P, Struhl K et al. Methylation of H3-lysine 79 is mediated by a new family of HMTases without a SET domain. Curr Biol 2002; 12: 1052–1058.
van Leeuwen F, Gafken PR, Gottschling DE . Dot1p modulates silencing in yeast by methylation of the nucleosome core. Cell 2002; 109: 745–756.
Shanower GA, Muller M, Blanton JL, Honti V, Gyurkovics H, Schedl P . Characterization of the grappa gene, the Drosophila histone H3 lysine 79 methyltransferase. Genetics 2005; 169: 173–184.
Nguyen AT, Zhang Y . The diverse functions of Dot1 and H3K79 methylation. Genes Dev 2011; 25: 1345–1358.
Song K, Nam YJ, Luo X, Qi X, Tan W, Huang GN et al. Heart repair by reprogramming non-myocytes with cardiac transcription factors. Nature 2012; 485: 599–604.
Epstein JA, Franklin H . Epstein lecture. Cardiac development and implications for heart disease. New Engl J Med 2010; 363: 1638–1647.
Keller GM . In vitro differentiation of embryonic stem cells. Curr Opin Cell Biol 1995; 7: 862–869.
Feng J, Liu T, Qin B, Zhang Y, Liu XS . Identifying ChIP-seq enrichment using MACS. Nat Protoc 2012; 7: 1728–1740.
Zang C, Schones DE, Zeng C, Cui K, Zhao K, Peng W . A clustering approach for identification of enriched domains from histone modification ChIP-Seq data. Bioinformatics 2009; 25: 1952–1958.
Zhu LJ, Gazin C, Lawson ND, Pages H, Lin SM, Lapointe DS et al. ChIPpeakAnno: a bioconductor package to annotate ChIP-seq and ChIP-chip data. BMC Bioinformatics 2010; 11: 237.
Heinz S, Benner C, Spann N, Bertolino E, Lin YC, Laslo P et al. Simple combinations of lineage-determining transcription factors prime cis-regulatory elements required for macrophage and B cell identities. Mol Cell 2010; 38: 576–589.
McLean CY, Bristor D, Hiller M, Clarke SL, Schaar BT, Lowe CB et al. GREAT improves functional interpretation of cis-regulatory regions. Nat Biotechnol 2010; 28: 495–501.
Robinson JT, Thorvaldsdottir H, Winckler W, Guttman M, Lander ES, Getz G et al. Integrative genomics viewer. Nat Biotechnol 2011; 29: 24–26.
Du P, Kibbe WA, Lin SM . lumi: a pipeline for processing Illumina microarray. Bioinformatics 2008; 24: 1547–1548.
Smyth GK, Michaud J, Scott HS . Use of within-array replicate spots for assessing differential expression in microarray experiments. Bioinformatics 2005; 21: 2067–2075.
Huang da W, Sherman BT, Lempicki RA . Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat Protoc 2009; 4: 44–57.
Acknowledgements
This work was supported by an ‘Advanced’ European Research Council (ERC) grant (Cardioepigen), grants from Fundation LeDucq and from MIUR (PRIN #2010RNXM9C_004) to CG, and from the Progetto Bandiera Epigenomica (EPIGEN) and the Progetto Bandiera Invecchiamento (Ageing Project) to PR.
Author Contributions
PC and PR designed the experiments, carried out research and wrote the manuscript; GC, RF, RR, PED, LSL and BC carried out research; KP, GA and SGG performed bioinformatics analyses; LMVG wrote the manuscript; CG supervised the whole research. All authors discussed the results and commented on the manuscript.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Edited by JC Marine
Supplementary Information accompanies this paper on Cell Death and Differentiation website
Rights and permissions
About this article
Cite this article
Cattaneo, P., Kunderfranco, P., Greco, C. et al. DOT1L-mediated H3K79me2 modification critically regulates gene expression during cardiomyocyte differentiation. Cell Death Differ 23, 555–564 (2016). https://doi.org/10.1038/cdd.2014.199
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/cdd.2014.199
This article is cited by
-
The disruptor of telomeric silencing 1-like (DOT1L) promotes peritoneal fibrosis through the upregulation and activation of protein tyrosine kinases
Molecular Biomedicine (2024)
-
Folate deficiency reduced aberrant level of DOT1L-mediated histone H3K79 methylation causes disruptive SHH gene expression involved in neural tube defects
Epigenetics & Chromatin (2023)
-
Nucleophosmin 1 cooperates with the methyltransferase DOT1L to preserve peri-nucleolar heterochromatin organization by regulating H3K27me3 levels and DNA repeats expression
Epigenetics & Chromatin (2023)
-
Inhibition of the cardiac fibroblast-enriched histone methyltransferase Dot1L prevents cardiac fibrosis and cardiac dysfunction
Cell & Bioscience (2022)
-
Allele-specific differential regulation of monoallelically expressed autosomal genes in the cardiac lineage
Nature Communications (2022)