Abstract
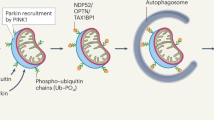
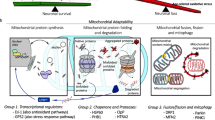
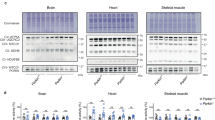
Mutations of the PARK2 and PINK1 genes, encoding the cytosolic E3 ubiquitin-protein ligase Parkin and the mitochondrial serine/threonine kinase PINK1, respectively, cause autosomal recessive early-onset Parkinson’s disease (PD). Parkin and PINK1 cooperate in a biochemical mitochondrial quality control pathway regulating mitochondrial morphology, dynamics and clearance. This study identifies the multifunctional PD-related mitochondrial matrix enzyme 17-β hydroxysteroid dehydrogenase type 10 (HSD17B10) as a new Parkin substrate. Parkin overproduction in cells increased mitochondrial HSD17B10 abundance by a mechanism involving ubiquitin chain extension, whereas PARK2 downregulation or deficiency caused mitochondrial HSD17B10 depletion in cells and mice. HSD17B10 levels were also found to be low in the brains of PD patients with PARK2 mutations. Confocal and Förster resonance energy transfer (FRET) microscopy revealed that HSD17B10 recruited Parkin to the translocase of the outer membrane (TOM), close to PINK1, both in functional mitochondria and after the collapse of mitochondrial membrane potential (ΔΨm). PD-causing PARK2 mutations impaired interaction with HSD17B10 and the HSD17B10-dependent mitochondrial translocation of Parkin. HSD17B10 overproduction promoted mitochondrial elongation and mitigated CCCP-induced mitochondrial degradation independently of enzymatic activity. These effects were abolished by overproduction of the fission-promiting dynamin-related protein 1 (Drp1). By contrast, siRNA-mediated HSD17B10 silencing enhanced mitochondrial fission and mitophagy. These findings suggest that the maintenance of appropriate mitochondrial HSD17B10 levels is one of the mechanisms by which Parkin preserves mitochondrial quality. The loss of this protective mechanism may contribute to mitochondrial dysfunction and neuronal degeneration in autosomal recessive PD.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
Abbreviations
- APEX:
-
pea L-ascorbate peroxidase (K14D, W41F, E112K) variant
- CCCP:
-
carbonyl cyanide m-chlorophenylhydrazone
- ΔΨm:
-
mitochondrial membrane potential
- DAB:
-
diaminobenzidine
- DLST:
-
dihydrolipoamide S-succinyl transferase
- Dox:
-
doxycycline
- Drp1:
-
Dynamin-related protein 1
- FRET:
-
Förster’s resonance energy transfer
- HSD17B10:
-
17-β hydroxysteroid dehydrogenase type 10
- OMM:
-
outer mitochondrial membrane
- MM:
-
mitochondrial membrane
- PINK1:
-
PTEN-induced putative kinase 1
- PMPCB:
-
mitochondrial processing peptidase subunit B
- TOM:
-
translocase of the outer membrane
- VDAC1:
-
voltage-dependent anion channel 1
References
Corti O, Lesage S, Brice A . What genetics tells us about the causes and mechanisms of Parkinson's disease. Physiol Rev 2011; 91: 1161–1218.
Byrd RA, Weissman AM . Compact Parkin only: insights into the structure of an autoinhibited ubiquitin ligase. EMBO J 2013; 32: 2087–2089.
Clark IE, Dodson MW, Jiang C, Cao JH, Huh JR, Seol JH et al. Drosophila pink1 is required for mitochondrial function and interacts genetically with parkin. Nature 2006; 441: 1162–1166.
Park J, Lee SB, Lee S, Kim Y, Song S, Kim S et al. Mitochondrial dysfunction in Drosophila PINK1 mutants is complemented by parkin. Nature 2006; 441: 1157–1161.
Deng H, Dodson MW, Huang H, Guo M . The Parkinson's disease genes pink1 and parkin promote mitochondrial fission and/or inhibit fusion in Drosophila. Proc Natl Acad Sci USA 2008; 105: 14503–14508.
Poole AC, Thomas RE, Andrews LA, McBride HM, Whitworth AJ, Pallanck LJ . The PINK1/Parkin pathway regulates mitochondrial morphology. Proc Natl Acad Sci USA 2008; 105: 1638–1643.
Yang Y, Ouyang Y, Yang L, Beal MF, McQuibban A, Vogel H et al. Pink1 regulates mitochondrial dynamics through interaction with the fission/fusion machinery. Proc Natl Acad Sci USA 2008; 105: 7070–7075.
Narendra D, Tanaka A, Suen DF, Youle RJ . Parkin is recruited selectively to impaired mitochondria and promotes their autophagy. J Cell Biol 2008; 183: 795–803.
Geisler S, Holmstrom KM, Skujat D, Fiesel FC, Rothfuss OC, Kahle PJ et al. PINK1/Parkin-mediated mitophagy is dependent on VDAC1 and p62/SQSTM1. Nat Cell Biol 2010; 12: 119–131.
Matsuda N, Sato S, Shiba K, Okatsu K, Saisho K, Gautier CA et al. PINK1 stabilized by mitochondrial depolarization recruits Parkin to damaged mitochondria and activates latent Parkin for mitophagy. J Cell Biol 2010; 189: 211–221.
Narendra DP, Jin SM, Tanaka A, Suen DF, Gautier CA, Shen J et al. PINK1 is selectively stabilized on impaired mitochondria to activate Parkin. PLoS Biol 2010; 8: e1000298.
Vives-Bauza C, Zhou C, Huang Y, Cui M, de Vries RL, Kim J et al. PINK1-dependent recruitment of Parkin to mitochondria in mitophagy. Proc Natl Acad Sci USA 2010; 107: 378–383.
Tanaka A, Cleland MM, Xu S, Narendra DP, Suen DF, Karbowski M et al. Proteasome and p97 mediate mitophagy and degradation of mitofusins induced by Parkin. J Cell Biol 2010; 191: 1367–1380.
Chan NC, Salazar AM, Pham AH, Sweredoski MJ, Kolawa NJ, Graham RL et al. Broad activation of the ubiquitin-proteasome system by Parkin is critical for mitophagy. Hum Mol Genet 2011; 20: 1726–1737.
Yoshii SR, Kishi C, Ishihara N, Mizushima N . Parkin mediates proteasome-dependent protein degradation and rupture of the outer mitochondrial membrane. J Biol Chem 2011; 286: 19630–19640.
Okatsu K, Iemura S, Koyano F, Go E, Kimura M, Natsume T et al. Mitochondrial hexokinase HKI is a novel substrate of the Parkin ubiquitin ligase. Biochem Biophys Res Commun 2012; 428: 197–202.
Sun Y, Vashisht AA, Tchieu J, Wohlschlegel JA, Dreier L . Voltage-dependent anion channels (VDACs) recruit Parkin to defective mitochondria to promote mitochondrial autophagy. J Biol Chem 2012; 287: 40652–40660.
Sarraf SA, Raman M, Guarani-Pereira V, Sowa ME, Huttlin EL, Gygi SP et al. Landscape of the PARKIN-dependent ubiquitylome in response to mitochondrial depolarization. Nature 2013; 496: 372–376.
McCoy MK, Kaganovich A, Rudenko IN, Ding J, Cookson MR . Hexokinase activity is required for recruitment of parkin to depolarized mitochondria. Hum Mol Genet 2014; 23: 145–156.
Chen Y, Dorn GW 2nd . PINK1-phosphorylated mitofusin 2 is a Parkin receptor for culling damaged mitochondria. Science 2013; 340: 471–475.
Lazarou M, Jin SM, Kane LA, Youle RJ . Role of PINK1 binding to the TOM complex and alternate intracellular membranes in recruitment and activation of the E3 ligase Parkin. Dev Cell 2012; 22: 320–333.
Bertolin G, Ferrando-Miguel R, Jacoupy M, Traver S, Grenier K, Greene AW et al. The TOMM machinery is a molecular switch in PINK1 and PARK2/PARKIN-dependent mitochondrial clearance. Autophagy 2013; 9: 1801–1817.
Darios F, Corti O, Lucking CB, Hampe C, Muriel MP, Abbas N et al. Parkin prevents mitochondrial swelling and cytochrome c release in mitochondria-dependent cell death. Hum Mol Genet 2003; 12: 517–526.
Zschocke J . HSD10 disease: clinical consequences of mutations in the HSD17B10 gene. J Inherit Metab Dis 2012; 35: 81–89.
Yang SY, He XY, Isaacs C, Dobkin C, Miller D, Philipp M . Roles of 17beta-hydroxysteroid dehydrogenase type 10 in neurodegenerative disorders. J Steroid Biochem Mol Biol 2014; 143: 460–472.
Rauschenberger K, Scholer K, Sass JO, Sauer S, Djuric Z, Rumig C et al. A non-enzymatic function of 17beta-hydroxysteroid dehydrogenase type 10 is required for mitochondrial integrity and cell survival. EMBO Mol Med 2010; 2: 51–62.
Zschocke J, Ruiter JP, Brand J, Lindner M, Hoffmann GF, Wanders RJ et al. Progressive infantile neurodegeneration caused by 2-methyl-3-hydroxybutyryl-CoA dehydrogenase deficiency: a novel inborn error of branched-chain fatty acid and isoleucine metabolism. Pediatr Res 2000; 48: 852–855.
Holzmann J, Frank P, Loffler E, Bennett KL, Gerner C, Rossmanith W . RNase P without RNA: identification and functional reconstitution of the human mitochondrial tRNA processing enzyme. Cell 2008; 135: 462–474.
Yan SD, Fu J, Soto C, Chen X, Zhu H, Al-Mohanna F et al. An intracellular protein that binds amyloid-beta peptide and mediates neurotoxicity in Alzheimer's disease. Nature 1997; 389: 689–695.
Lustbader JW, Cirilli M, Lin C, Xu HW, Takuma K, Wang N et al. ABAD directly links Abeta to mitochondrial toxicity in Alzheimer's disease. Science 2004; 304: 448–452.
Tieu K, Perier C, Vila M, Caspersen C, Zhang HP, Teismann P et al. L-3-hydroxyacyl-CoA dehydrogenase II protects in a model of Parkinson's disease. Ann Neurol 2004; 56: 51–60.
Goldberg MS, Fleming SM, Palacino JJ, Cepeda C, Lam HA, Bhatnagar A et al. Parkin-deficient mice exhibit nigrostriatal deficits but not loss of dopaminergic neurons. J Biol Chem 2003; 278: 43628–43635.
Itier JM, Ibanez P, Mena MA, Abbas N, Cohen-Salmon C, Bohme GA et al. Parkin gene inactivation alters behaviour and dopamine neurotransmission in the mouse. Hum Mol Genet 2003; 12: 2277–2291.
Palacino JJ, Sagi D, Goldberg MS, Krauss S, Motz C, Wacker M et al. Mitochondrial dysfunction and oxidative damage in parkin-deficient mice. J Biol Chem 2004; 279: 18614–18622.
Von Coelln R, Thomas B, Savitt JM, Lim KL, Sasaki M, Hess EJ et al. Loss of locus coeruleus neurons and reduced startle in parkin null mice. Proc Natl Acad Sci USA 2004; 101: 10744–10749.
Perez FA, Palmiter RD . Parkin-deficient mice are not a robust model of parkinsonism. Proc Natl Acad Sci USA 2005; 102: 2174–2179.
Periquet M, Corti O, Jacquier S, Brice A . Proteomic analysis of parkin knockout mice: alterations in energy metabolism, protein handling and synaptic function. J Neurochem 2005; 95: 1259–1276.
Damiano M, Gautier CA, Bulteau AL, Ferrando-Miguel R, Gouarne C, Paoli MG et al. Tissue- and cell-specific mitochondrial defect in Parkin-deficient mice. PLoS One 2014; 9: e99898.
Greene AW, Grenier K, Aguileta MA, Muise S, Farazifard R, Haque ME et al. Mitochondrial processing peptidase regulates PINK1 processing, import and Parkin recruitment. EMBO Rep 2012; 13: 378–385.
Jin SM, Youle RJ . The accumulation of misfolded proteins in the mitochondrial matrix is sensed by PINK1 to induce PARK2/Parkin-mediated mitophagy of polarized mitochondria. Autophagy 2013; 9: 1750–1757.
Rojo M, Legros F, Chateau D, Lombes A . Membrane topology and mitochondrial targeting of mitofusins, ubiquitous mammalian homologs of the transmembrane GTPase Fzo. J Cell Sci 2002; 115: 1663–1674.
Chacinska A, Koehler CM, Milenkovic D, Lithgow T, Pfanner N . Importing mitochondrial proteins: machineries and mechanisms. Cell 2009; 138: 628–644.
Snapp EL, Hegde RS . Rational design and evaluation of FRET experiments to measure protein proximities in cells. Curr Protoc Cell Biol 2006; Chapter 17: Unit 17 19.
Koopman WJ, Visch HJ, Verkaart S, van den Heuvel LW, Smeitink JA, Willems PH . Mitochondrial network complexity and pathological decrease in complex I activity are tightly correlated in isolated human complex I deficiency. Am J Physiol Cell Physiol 2005; 289: C881–C890.
Detmer SA, Chan DC . Functions and dysfunctions of mitochondrial dynamics. Nat Rev Mol Cell Biol 2007; 8: 870–879.
Twig G, Elorza A, Molina AJ, Mohamed H, Wikstrom JD, Walzer G et al. Fission and selective fusion govern mitochondrial segregation and elimination by autophagy. EMBO J 2008; 27: 433–446.
Buhlman L, Damiano M, Bertolin G, Ferrando-Miguel R, Lombes A, Brice A et al. Functional interplay between Parkin and Drp1 in mitochondrial fission and clearance. Biochim Biophys Acta 2014; 1843: 2012–2026.
Tondera D, Grandemange S, Jourdain A, Karbowski M, Mattenberger Y, Herzig S et al. SLP-2 is required for stress-induced mitochondrial hyperfusion. EMBO J 2009; 28: 1589–1600.
Gomes LC, Di Benedetto G, Scorrano L . During autophagy mitochondria elongate, are spared from degradation and sustain cell viability. Nat Cell Biol 2011; 13: 589–598.
Rambold AS, Kostelecky B, Elia N, Lippincott-Schwartz J . Tubular network formation protects mitochondria from autophagosomal degradation during nutrient starvation. Proc Natl Acad Sci USA 2011; 108: 10190–10195.
Cereghetti GM, Stangherlin A, Martins de Brito O, Chang CR, Blackstone C, Bernardi P et al. Dephosphorylation by calcineurin regulates translocation of Drp1 to mitochondria. Proc Natl Acad Sci USA 2008; 105: 15803–15808.
Joselin AP, Hewitt SJ, Callaghan SM, Kim RH, Chung YH, Mak TW et al. ROS-dependent regulation of Parkin and DJ-1 localization during oxidative stress in neurons. Hum Mol Genet 2012; 21: 4888–4903.
Bragoszewski P, Gornicka A, Sztolsztener ME, Chacinska A . The ubiquitin-proteasome system regulates mitochondrial intermembrane space proteins. Mol Cell Biol 2013; 33: 2136–2148.
Hawrylycz MJ, Lein ES, Guillozet-Bongaarts AL, Shen EH, Ng L, Miller JA et al. An anatomically comprehensive atlas of the adult human brain transcriptome. Nature 2012; 489: 391–399.
Vilardo E, Nachbagauer C, Buzet A, Taschner A, Holzmann J, Rossmanith W . A subcomplex of human mitochondrial RNase P is a bifunctional methyltransferase—extensive moonlighting in mitochondrial tRNA biogenesis. Nucleic Acids Res 2012; 40: 11583–11593.
Muller-Rischart AK, Pilsl A, Beaudette P, Patra M, Hadian K, Funke M et al. The E3 ligase parkin maintains mitochondrial integrity by increasing linear ubiquitination of NEMO. Mol Cell 2013; 49: 908–921.
Hampe C, Ardila-Osorio H, Fournier M, Brice A, Corti O . Biochemical analysis of Parkinson's disease-causing variants of Parkin, an E3 ubiquitin-protein ligase with monoubiquitylation capacity. Hum Mol Genet 2006; 15: 2059–2075.
Martell JD, Deerinck TJ, Sancak Y, Poulos TL, Mootha VK, Sosinsky GE et al. Engineered ascorbate peroxidase as a genetically encoded reporter for electron microscopy. Nat Biotechnol 2012; 30: 1143–1148.
Fallon L, Belanger CM, Corera AT, Kontogiannea M, Regan-Klapisz E, Moreau F et al. A regulated interaction with the UIM protein Eps15 implicates parkin in EGF receptor trafficking and PI(3)K-Akt signalling. Nat Cell Biol 2006; 8: 834–842.
Escobar-Khondiker M, Hollerhage M, Muriel MP, Champy P, Bach A, Depienne C et al. Annonacin, a natural mitochondrial complex I inhibitor, causes tau pathology in cultured neurons. J Neurosci 2007; 27: 7827–7837.
Acknowledgements
We thank M Rojo and A Lombès for helpful discussions, S Jacquier for technical assistance, the Plate-Forme d’Imagerie Cellulaire de la Pitié-Salpêtrière (PICPS), G Kroemer Y, Z Ronai, Y Yarden, M Rojo, J Zschocke for providing vectors, and the Queen Square Brain Bank (QSBB, Institute of Neurology, London) for providing human brain tissue. This work was supported by Institut national de la santé et de la recherche médicale, Association France Parkinson, Fondation de France (Engt 2007 006456, Engt 2012 00034508), Agence Nationale de la Recherche (ANR-08-MNPS-02-01) APOPIS (funded by the EU under the Sixth Framework Programme, Grant Agreement LSHM-CT-2003-503330), MEFOPA (funded by the EU under the 7th Framework Programme, Grant Agreement HEALTH-2009-241791), Fondation ICM, ‘Investissements d’avenir’ ANR-10-IAIHU-06. GB and MJ were supported by a fellowship from the French Ministry of Higher Education and Research. GB was also supported by Fondation de France.
Author Contributions
GB and MJ designed, performed and analyzed the experiments and wrote the manuscript; ST designed, performed and analyzed the experiments; RF-M, HA-O, TSG and M-PM performed and analyzed the experiments; HT and AJL provided neuropathologically characterized human brain tissue; FC, CG and DG designed and provided APEX constructs and advice; KG and EAF performed the experiments, shared protocols and provided advice; AB jointly supervised the work and provided funding; OC supervised and coordinated the work, provided funding and wrote the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Edited by L Scorrano
Supplementary Information accompanies this paper on Cell Death and Differentiation website
Supplementary information
Rights and permissions
About this article
Cite this article
Bertolin, G., Jacoupy, M., Traver, S. et al. Parkin maintains mitochondrial levels of the protective Parkinson’s disease-related enzyme 17-β hydroxysteroid dehydrogenase type 10. Cell Death Differ 22, 1563–1576 (2015). https://doi.org/10.1038/cdd.2014.224
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/cdd.2014.224
This article is cited by
-
PINK1 deficiency impairs osteoblast differentiation through aberrant mitochondrial homeostasis
Stem Cell Research & Therapy (2021)
-
Connecting the dots between mitochondrial dysfunction and Parkinson’s disorder: focus mitochondria-targeting therapeutic paradigm in mitigating the disease severity
Environmental Science and Pollution Research (2021)
-
PINK1 and Parkin mitochondrial quality control: a source of regional vulnerability in Parkinson’s disease
Molecular Neurodegeneration (2020)
-
Deacetylation of HSD17B10 by SIRT3 regulates cell growth and cell resistance under oxidative and starvation stresses
Cell Death & Disease (2020)
-
Interactions of 17β-Hydroxysteroid Dehydrogenase Type 10 and Cyclophilin D in Alzheimer's Disease
Neurochemical Research (2020)