Abstract
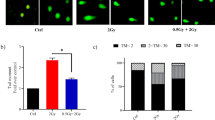
Because of insufficient understanding of the molecular effects of low levels of radiation exposure, there is a great uncertainty regarding its health risks. We report here that treatment of normal human cells with low-dose radiation induces a metabolic shift from oxidative phosphorylation to aerobic glycolysis resulting in increased radiation resistance. This metabolic change is highlighted by upregulation of genes encoding glucose transporters and enzymes of glycolysis and the oxidative pentose phosphate pathway, concomitant with downregulation of mitochondrial genes, with corresponding changes in metabolic flux through these pathways. Mechanistically, the metabolic reprogramming depends on HIF1α, which is induced specifically by low-dose irradiation linking the metabolic pathway with cellular radiation dose response. Increased glucose flux and radiation resistance from low-dose irradiation are also observed systemically in mice. This highly sensitive metabolic response to low-dose radiation has important implications in understanding and assessing the health risks of radiation exposure.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
Abbreviations
- CT:
-
computed tomography
- ECAR:
-
elevated extracellular acidification rate
- 2-DG:
-
2-deoxyglucose
- G6PD:
-
glucose-6-phosphate dehydrogenase
- GLUT:
-
glucose transporter
- HIF-1:
-
hypoxia induced factor
- IR:
-
ionizing radiation
- LDH:
-
lactate dehydrogenase
- LNT:
-
linear no-threshold
- LD50:
-
lethal dose of 50%
- MCT:
-
monocarboxylate transporter
- PPP:
-
pentose phosphate pathway
- ROS:
-
reactive oxygen species
- SOD:
-
superoxide dismutase
- TCA:
-
tricarboxylic acid cycle
References
Mobbs SF, Muirhead CR, Harrison JD . Risks from ionising radiation: an HPA viewpoint paper for Safegrounds. J Radiol Prot 2011; 31: 289–307.
Siegel JA, Stabin MG . Radar commentary: use of linear no-threshold hypothesis in radiation protection regulation in the United States. Health Phys 2010; 102: 90–99.
Mossman KS . Policy decision-making under scientific uncertainty: radiological risk assessment and the role of expert advisory groups. Health Phys 2009; 97: 101–106.
Vaiserman AM . Radiation hormesis: historical perspective and implications for low-dose cancer risk assessment. Dose Response 2010; 8: 172–191.
Calabrese EJ . The road to linearity, why linearity at low doses became the basis for carcinogen risk assessment. Arch Toxical 2009; 83: 203–225.
Mitchel RE . The dose window for radiation-induced protective adaptive responses. Dose Response 2010; 8: 192–208.
Morgan WF, Bair WJ . Issues in low-dose radiation biology: the controversy continues. A perspective. Radiat Res 2013; 79: 501–510.
Mullenders L, Atkinson M, Paretzke H, Sabatier l, Bouffler S . Assessing cancer risks of low-dose radiation. Nat Rev Cancer 2009; 9: 596–604.
Hall E, Giaccia AJ . Radiobiology for the Radiobiologist. Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2006.
Finkel T . Signal transduction by mitochondrial oxidants. JBC 2012; 287: 4434–4440.
Sena LA, Chandel NS . Physiological roles of mitochondrial reactive oxygen species. Mol Cell 2012; 48: 158–167.
Rhee SG, Bae YS, Lee SR, Kwon J . Hydrogen peroxide: a key messenger that modulates protein phosphorylation through cysteine oxidation. Sci STKE 2000; 53: pe1.
Tonks NK . Redox redux: revisiting PTPs and the control of cell signaling. Cell 2005; 121: 667–670.
Goran JD, Simon MC . Hypoxia-inducible factors, central regulators of the tumor phenotype. Curr Opom Genet Dev 2007; 17: 71–77.
Semenza GL . Hypoxia-inducible factors in physiology and medicine. Cell 2012; 148: 399–408.
Van Uden P, Kenneth NS, Rocha S . Regulation of hypoxia-inducible factor-1α by NF-κB. Biochem J 2008; 412: 477–484.
Rois J, Guma M, Schachtrup C, Akassoglou K, Zinkernagel AS, Nizet V et al. NF-κB links innate immunity to the hypoxic response through transcriptional regulation of HIF-1α. Nature 2008; 453: 807–811.
Pan Y, Mansfield KD, Bertozzi CC, Rudenko V, Chan DA, Giaccia AJ et al. Multiple factors affecting cellular redox status and energy metabolism modulate hypoxia-inducible factor prolyl hydroxylase activity in vivo and in vitro. Mol Cell Biol 2007; 27: 912–925.
Koppenol WH, Bounds PL, Dang CV . Otto Warburg's contributions to current concepts of cancer metabolism. Nat Rev Cancer 2011; 11: 325–337.
Lunt SY, Vancer Heiden MG . Aerobic glycolysis: meeting the metabolic requirement of cell proliferation. Annu Rev Cell Dev Biol. 2011; 27: 441–464.
Chan SY, Zhang YY, Hemann C, Mahoney CE, Zweier JL, Loscalzo J . MicroRNA-210 controls mitochondrial metabolism during hypoxia by repressing the iron-sulfur cluster assembly proteins ISCU1/2. Cell Metab 2009; 10: 273–284.
Corn PG . Hypoxic regulation of miR-210: shrinking targets expand HIF-1s influence. Cancer Biol Ther 2008; 7: 265–267.
Kastan MB, Onyekwere O, Sidransky D, Vogelstein B, Craig RW . Participation of p53 protein in the cellular response to DNA damage. Cancer Res 1991; 51: 6304–6311.
Mole RH . The LD50 for uniform low LET irradiation of man. Br J Radiol 1984; 57: 355–369.
Ward JP . Oxygen sensors in context. Biochimica et Biophsica Acta 2008; 1777: 1–14.
Halestrap AP, Price NT . The proton-linked monocarboxylate transporter (MCT) family: structure, function and regulation. Biochem J 1999; 343: 281–299.
Yuan M, Breitkopf SB, Yang X, Asara JM . A positive/negative ion-switching, targeted mass spectrometry-based metabolomics platform for bodily fluids, cells, and fresh and fixed tissue. Nat Protoc 2012; 7: 872–881.
Ben-Sahra I, Howell JJ, Asara JM, Manning BD . Stimulation of de novo pyrimidine synthesis by growth signaling through mTOR and S6K1. Science 2013; 339: 1323–13287.
Fantin VR, St-Pierre J, Leder P . Attenuation of LDH-A expression uncovers a link between glycolysis, mitochondrial physiology, and tumor maintenance. Cancer Cell 2006; 9: 425–434.
Aylon Y, Oren M . New plays in the p53 theater. Curr Opin Genet Dev 2011; 21: 86–92.
Vousden KH, Prives C . Blinded by the light, the growing complexity of p53. Cell 2009; 37: 413–431.
Jackson JG, Post SM, Lozano G . Regulation of tissue- and stimulus-specific cell fate decisions by p53 in vivo. J Pathol 2011; 223: 127–136.
Gudkov AV, Komarova EA . The role of p53 in determining sensitivity to radiotherapy. Nat Rev Cancer 2003; 3: 117–129.
Wang T, Marquardt C, Foker J . Aerobic glycolysis during lymphocyte-proliferation. Nature 1976; 261: 702–705.
Ben-Neriah Y, Karin M . Inflammation meets cancer, with NF-kappaB as the matchmaker. Nat Immunol 2011; 12: 715–723.
Huang Y, Zhang J, McHenry KT, Kim MM, Zeng W, Lopez-Pajares V et al. Induction of cytoplasmic accumulation of p53: a mechanism for low levels of arsenic exposure to predispose cells for malignant transformation. Cancer Res 2008; 68: 9131–9136.
Wiederschain D, Kawai H, Gu J, Shilatifard A, Yuan ZM . Molecular basis of p53 functional inactivation by the leukemic protein MLL-ELL. Mol Cell Biol 2003; 23: 4230–4246.
Acknowledgements
We would like to thank the UTHSCSA Small Animal Image Core, Suresh Prajapati for animal imaging studies, Min Yuan for help with mass spectrometry analyses and Drs. Hotamisligil & Fu at HSPH for the XF Analyzer analysis. These studies were supported by the Morningside Foundation, the Department of Energy (DOE 110976 and 65089), NIH/NCI (2 R01CA085679, RO1CA167814, RO1CA125144, and P01CA120964).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Edited by N Chandel
Author contributions
ZY designed the experiments. RL, TX, HS, SX and MS contributed to cell-based studies. MY performed gene expression experiments. SG and CSH did in vivo work. JMA, IB-S, BDM and JBL planed and conducted metabolic analyses. Z-MY wrote and BDM and JBL edited the manuscript.
Supplementary Information accompanies this paper on Cell Death and Differentiation website
Supplementary information
Rights and permissions
About this article
Cite this article
Lall, R., Ganapathy, S., Yang, M. et al. Low-dose radiation exposure induces a HIF-1-mediated adaptive and protective metabolic response. Cell Death Differ 21, 836–844 (2014). https://doi.org/10.1038/cdd.2014.24
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/cdd.2014.24
This article is cited by
-
Mesenchymal Stem Cells and Selenium Nanoparticles Synergize with Low Dose of Gamma Radiation to Suppress Mammary Gland Carcinogenesis via Regulation of Tumor Microenvironment
Biological Trace Element Research (2023)
-
Functional interplay between p53 and Δ133p53 in adaptive stress response
Cell Death & Differentiation (2020)
-
Disturbance in the regulation of miR 17-92 cluster on HIF-1-α expression contributes to clinically relevant radioresistant cells: an in vitro study
Cytotechnology (2020)
-
Radiation Therapy Reduced Blood Levels of LDH, HIF-1α, and miR-210 in OSCC
Pathology & Oncology Research (2020)
-
p53/BNIP3-dependent mitophagy limits glycolytic shift in radioresistant cancer
Oncogene (2019)