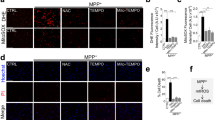
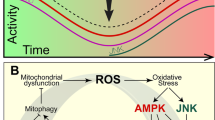
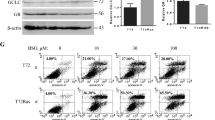
Abstract
Reactive oxygen species (ROS) are well known to be involved in oncogene-mediated cellular transformation. However, the regulatory mechanisms underlying ROS generation in oncogene-transformed cells are unclear. In the present study, we found that oncogenic K-Ras induces ROS generation through activation of NADPH oxidase 1 (NOX1), which is a critical regulator for the K-Ras-induced cellular transformation. NOX1 was activated by K-Ras-dependent translocation of p47phox, a subunit of NOX1 to plasma membrane. Of note, PKCδ, when it was activated by PDPK1, directly bound to the SH3-N domain of p47phox and catalyzed the phosphorylation on Ser348 and Ser473 residues of p47phox C-terminal in a K-Ras-dependent manner, finally leading to its membrane translocation. Notably, oncogenic K-Ras activated all MAPKs (JNK, ERK and p38); however, only p38 was involved in p47phox-NOX1-dependent ROS generation and consequent transformation. Importantly, K-Ras-induced activation of p38 led to an activation of PDPK1, which then signals through PKCδ, p47phox and NOX1. In agreement with the mechanism, inhibition of p38, PDPK1, PKCδ, p47phox or NOX1 effectively blocked K-Ras-induced ROS generation, anchorage-independent colony formation and tumor formation. Taken together, our findings demonstrated that oncogenic K-Ras activates the signaling cascade p38/PDPK1/PKCδ/p47phox/NOX1 for ROS generation and consequent malignant cellular transformation.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
Abbreviations
- PKC:
-
protein kinase C
- PDPK1:
-
3-phosphoinositide-dependent protein kinase-1
- MAPK:
-
mitogen-activated protein kinase
- NADPH:
-
nicotinamide adenine dinucleotide phosphate-oxidase
- DN:
-
dominant-negative mutant form
- ROS:
-
reactive oxygen species
- GST:
-
glutathione S-transferases
- siRNA:
-
small interfering RNA
- WT:
-
wild type
References
Sundaresan M, Yu ZX, Ferrans VJ, Irani K, Finkel T . Requirement for generation of H2O2 for platelet-derived growth factor signal transduction. Science 1995; 270: 296–299.
Bae YS, Kang SW, Seo MS, Baines IC, Tekle E, Chock PB et al. Epidermal growth factor (EGF)-induced generation of hydrogen peroxide. Role in EGF receptor-mediated tyrosine phosphorylation. J Biol Chem 1997; 272: 217–221.
Leto TL, Geiszt M . Role of Nox family NADPH oxidases in host defense. Antioxid Redox Signal 2006; 8: 1549–1561.
Thannickal VJ, Fanburg BL . Reactive oxygen species in cell signaling. Am J Physiol Lung Cell Mol Physiol 2000; 279: L1005–L1028.
Orient A, Donko A, Szabo A, Leto TL, Geiszt M . Novel sources of reactive oxygen species in the human body. Nephrol Dial Transplant 2007; 22: 1281–1288.
Halliwell B, Gutteridge JM, Cross CE . Free radicals, antioxidants, and human disease: where are we now? J Lab Clin Med 1992; 119: 598–620.
Cross CE, Halliwell B, Borish ET, Pryor WA, Ames BN, Saul RL et al. Oxygen radicals and human disease. Ann Intern Med 1987; 107: 526–545.
Kim C, Kim JY, Kim JH . Cytosolic phospholipase A(2), lipoxygenase metabolites, and reactive oxygen species. BMB Rep 2008; 41: 555–559.
Vafa O, Wade M, Kern S, Beeche M, Pandita TK, Hampton GM et al. c-Myc can induce DNA damage, increase reactive oxygen species, and mitigate p53 function: a mechanism for oncogene-induced genetic instability. Mol Cell 2002; 9: 1031–1044.
Gianni D, Taulet N, DerMardirossian C, Bokoch GM . c-Src-mediated phosphorylation of NoxA1 and Tks4 induces the reactive oxygen species (ROS)-dependent formation of functional invadopodia in human colon cancer cells. Mol Biol Cell 2010; 21: 4287–4298.
Ferro E, Goitre L, Retta SF, Trabalzini L . The interplay between ROS and Ras GTPases: physiological and pathological implications. J Signal Transduct 2012; 2012: 365769.
Yang JQ, Li S, Huang Y, Zhang HJ, Domann FE, Buettner GR et al. V-Ha-Ras overexpression induces superoxide production and alters levels of primary antioxidant enzymes. Antioxid Redox Signal 2001; 3: 697–709.
Bos JL . ras oncogenes in human cancer: a review. Cancer Res 1989; 49: 4682–4689.
Adjei AA . Blocking oncogenic Ras signaling for cancer therapy. J Natl Cancer Inst 2001; 93: 1062–1074.
Yang JQ, Li S, Domann FE, Buettner GR, Oberley LW . Superoxide generation in v-Ha-ras-transduced human keratinocyte HaCaT cells. Mol Carcinog 1999; 26: 180–188.
Yang JQ, Buettner GR, Domann FE, Li Q, Engelhardt JF, Weydert CD et al. v-Ha-ras mitogenic signaling through superoxide and derived reactive oxygen species. Mol Carcinog 2002; 33: 206–218.
Cullen JJ, Weydert C, Hinkhouse MM, Ritchie J, Domann FE, Spitz D et al. The role of manganese superoxide dismutase in the growth of pancreatic adenocarcinoma. Cancer Res 2003; 63: 1297–1303.
Ang KK, Peters LJ, Weber RS, Morrison WH, Frankenthaler RA, Garden AS et al. Postoperative radiotherapy for cutaneous melanoma of the head and neck region. Int J Radiat Oncol Biol Phys 1994; 30: 795–798.
Maciag A, Sithanandam G, Anderson LM . Mutant K-rasV12 increases COX-2, peroxides and DNA damage in lung cells. Carcinogenesis 2004; 25: 2231–2237.
Ralph SJ, Rodriguez-Enriquez S, Neuzil J, Saavedra E, Moreno-Sanchez R . The causes of cancer revisited: ‘mitochondrial malignancy’ and ROS-induced oncogenic transformation—why mitochondria are targets for cancer therapy. Mol Aspects Med 2010; 31: 145–170.
Mitsushita J, Lambeth JD, Kamata T . The superoxide-generating oxidase Nox1 is functionally required for Ras oncogene transformation. Cancer Res 2004; 64: 3580–3585.
Cheng G, Lambeth JD . NOXO1, regulation of lipid binding, localization, and activation of Nox1 by the Phox homology (PX) domain. J Biol Chem 2004; 279: 4737–4742.
Ago T, Kuribayashi F, Hiroaki H, Takeya R, Ito T, Kohda D et al. Phosphorylation of p47phox directs phox homology domain from SH3 domain toward phosphoinositides, leading to phagocyte NADPH oxidase activation. Proc Natl Acad Sci USA 2003; 100: 4474–4479.
Yuzawa S, Suzuki NN, Fujioka Y, Ogura K, Sumimoto H, Inagaki F . A molecular mechanism for autoinhibition of the tandem SH3 domains of p47phox, the regulatory subunit of the phagocyte NADPH oxidase. Genes Cells 2004; 9: 443–456.
Symonds JM, Ohm AM, Carter CJ, Heasley LE, Boyle TA, Franklin WA et al. Protein kinase C delta is a downstream effector of oncogenic K-ras in lung tumors. Cancer Res 2011; 71: 2087–2097.
Pylayeva-Gupta Y, Grabocka E, Bar-Sagi D . RAS oncogenes: weaving a tumorigenic web. Nat Rev Cancer 2011; 11: 761–774.
Avruch J, Khokhlatchev A, Kyriakis JM, Luo Z, Tzivion G, Vavvas D et al. Ras activation of the Raf kinase: tyrosine kinase recruitment of the MAP kinase cascade. Recent Prog Horm Res 2001; 56: 127–155.
Welman A, Griffiths JR, Whetton AD, Dive C . Protein kinase C delta is phosphorylated on five novel Ser/Thr sites following inducible overexpression in human colorectal cancer cells. Protein Sci 2007; 16: 2711–2715.
Parekh DB, Ziegler W, Parker PJ . Multiple pathways control protein kinase C phosphorylation. Embo J 2000; 19: 496–503.
Stephens L, Anderson K, Stokoe D, Erdjument-Bromage H, Painter GF, Holmes AB et al. Protein kinase B kinases that mediate phosphatidylinositol 3,4,5-trisphosphate-dependent activation of protein kinase B. Science 1998; 279: 710–714.
Alessi DR, James SR, Downes CP, Holmes AB, Gaffney PR, Reese CB et al. Characterization of a 3-phosphoinositide-dependent protein kinase which phosphorylates and activates protein kinase Balpha. Curr Biol 1997; 7: 261–269.
Stokoe D, Stephens LR, Copeland T, Gaffney PR, Reese CB, Painter GF et al. Dual role of phosphatidylinositol-3,4,5-trisphosphate in the activation of protein kinase B. Science 1997; 277: 567–570.
Le Good JA, Ziegler WH, Parekh DB, Alessi DR, Cohen P, Parker PJ . Protein kinase C isotypes controlled by phosphoinositide 3-kinase through the protein kinase PDK1. Science 1998; 281: 2042–2045.
Hata K, Ito T, Takeshige K, Sumimoto H . Anionic amphiphile-independent activation of the phagocyte NADPH oxidase in a cell-free system by p47phox and p67phox, both in C terminally truncated forms. Implication for regulatory Src homology 3 domain-mediated interactions. J Biol Chem 1998; 273: 4232–4236.
Nitti M, Furfaro AL, Cevasco C, Traverso N, Marinari UM, Pronzato MA et al. PKC delta and NADPH oxidase in retinoic acid-induced neuroblastoma cell differentiation. Cell Signal 2010; 22: 828–835.
Nitti M, Furfaro AL, Traverso N, Odetti P, Storace D, Cottalasso D et al. PKC delta and NADPH oxidase in AGE-induced neuronal death. Neurosci Lett 2007; 416: 261–265.
Cai W, Torreggiani M, Zhu L, Chen X, He JC, Striker GE et al. AGER1 regulates endothelial cell NADPH oxidase-dependent oxidant stress via PKC-delta: implications for vascular disease. AmJ Physiol Cell Physiol 2010; 298: C624–C634.
Raimondi C, Falasca M . Targeting PDK1 in cancer. Curr Med Chem 2011; 18: 2763–2769.
Xie Z, Yuan H, Yin Y, Zeng X, Bai R, Glazer RI . 3-phosphoinositide-dependent protein kinase-1 (PDK1) promotes invasion and activation of matrix metalloproteinases. BMC Cancer 2006; 6: 77.
Eser S, Reiff N, Messer M, Seidler B, Gottschalk K, Dobler M et al. Selective requirement of PI3K/PDK1 signaling for Kras oncogene-driven pancreatic cell plasticity and cancer. Cancer Cell 2013; 23: 406–420.
Aoki Y, Niihori T, Narumi Y, Kure S, Matsubara Y . The RAS/MAPK syndromes: novel roles of the RAS pathway in human genetic disorders. Hum Mutat 2008; 29: 992–1006.
Molina JR, Adjei AA . The Ras/Raf/MAPK pathway. J Thorac Oncol 2006; 1: 7–9.
Kim RK, Suh Y, Lim EJ, Yoo KC, Lee GH, Cui YH et al. A novel 2-pyrone derivative, BHP, impedes oncogenic KRAS-driven malignant progression in breast cancer. Cancer Lett 2013; 337: 49–57.
Acknowledgements
This work was supported by the National Research Foundation (NRF) and Ministry of Science, ICT and Future Planning, Korean government, through its National Nuclear Technology Program (2012M2A2A7035878 and 2012M2B2B1055639).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Edited by N Chandel
Supplementary Information accompanies this paper on Cell Death and Differentiation website
Rights and permissions
About this article
Cite this article
Park, MT., Kim, MJ., Suh, Y. et al. Novel signaling axis for ROS generation during K-Ras-induced cellular transformation. Cell Death Differ 21, 1185–1197 (2014). https://doi.org/10.1038/cdd.2014.34
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/cdd.2014.34
This article is cited by
-
The role of ROS in tumour development and progression
Nature Reviews Cancer (2022)
-
Targeting de novo lipogenesis and the Lands cycle induces ferroptosis in KRAS-mutant lung cancer
Nature Communications (2022)
-
Senescent tumor cells: an overlooked adversary in the battle against cancer
Experimental & Molecular Medicine (2021)
-
Current updates on the role of reactive oxygen species in bladder cancer pathogenesis and therapeutics
Clinical and Translational Oncology (2020)
-
Melatonin: an endogenous miraculous indolamine, fights against cancer progression
Journal of Cancer Research and Clinical Oncology (2020)