Abstract
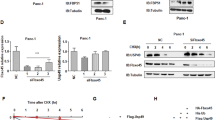
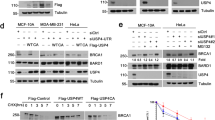
Prostate apoptosis response protein 4 (Par-4) also known as PRKC apoptosis WT1 regulator is a tumor suppressor that selectively induces apoptosis in cancer cells. However, its post-translational regulation by ubiquitin-mediated proteolysis and the cellular machinery that is responsible for its proteasomal degradation are unknown. Using immunopurification and an unbiased mass spectrometry-based approach, we show that Par-4 interacts with the SPRY-domain containing E3 ubiquitin ligase Fbxo45 through a short consensus sequence motif. Fbxo45 interacts with Par-4 in the cytoplasm and mediates its ubiquitylation and proteasomal degradation. Fbxo45 silencing results in stabilization of Par-4 with increased apoptosis. Importantly, a Par-4 mutant that is unable to bind Fbxo45 is stabilized and further enhances staurosporine-induced apoptosis. Co-expression of Fbxo45 with Par-4 protects cancer cells against Par-4-induced apoptosis. Our studies reveal that Fbxo45 is the substrate-receptor subunit of a functional E3 ligase for Par-4 that has a critical role in cancer cell survival.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
Abbreviations
- Par-4:
-
Prostate apoptosis response protein 4
- PAWR:
-
PRKC apoptosis WT1 regulator
- PAM:
-
protein-associated with myc
- SCF:
-
Skp1, CUL1, F-box protein
- MYCBP2:
-
myc-binding protein 2
- NLS:
-
nuclear localization sequence
- LC-MS/MS:
-
liquid chromatography-tandem mass spectrometry
- mES:
-
mouse embryonic stem
- SOCS:
-
suppressor of cytokine signaling
- WCE:
-
whole-cell extract
References
Bai C, Sen P, Hofmann K, Ma L, Goebl M, Harper JW et al. SKP1 connects cell cycle regulators to the ubiquitin proteolysis machinery through a novel motif, the F-box. Cell 1996; 86: 263–274.
Cenciarelli C, Chiaur DS, Guardavaccaro D, Parks W, Vidal M, Pagano M . Identification of a family of human F-box proteins. Curr Biol 1999; 9: 1177–1179.
Jin J, Cardozo T, Lovering RC, Elledge SJ, Pagano M, Harper JW . Systematic analysis and nomenclature of mammalian F-box proteins. Genes Dev 2004; 18: 2573–2580.
Skowyra D, Craig KL, Tyers M, Elledge SJ, Harper JW . F-box proteins are receptors that recruit phosphorylated substrates to the SCF ubiquitin-ligase complex. Cell 1997; 91: 209–219.
Vashisht AA, Zumbrennen KB, Huang X, Powers DN, Durazo A, Sun D et al. Control of iron homeostasis by an iron-regulated ubiquitin ligase. Science 2009; 326: 718–721.
Wertz IE, Kusam S, Lam C, Okamoto T, Sandoval W, Anderson DJ et al. Sensitivity to antitubulin chemotherapeutics is regulated by MCL1 and FBW7. Nature 2011; 471: 110–114.
Liao EH, Hung W, Abrams B, Zhen M . An SCF-like ubiquitin ligase complex that controls presynaptic differentiation. Nature 2004; 430: 345–350.
Saiga T, Fukuda T, Matsumoto M, Tada H, Okano HJ, Okano H et al. Fbxo45 forms a novel ubiquitin ligase complex and is required for neuronal development. Mol Cell Biol 2009; 29: 3529–3543.
Peschiaroli A, Scialpi F, Bernassola F, Pagano M, Melino G . The F-box protein FBXO45 promotes the proteasome-dependent degradation of p73. Oncogene 2009; 28: 3157–3166.
Tada H, Okano HJ, Takagi H, Shibata S, Yao I, Matsumoto M et al. Fbxo45, a novel ubiquitin ligase, regulates synaptic activity. J Biol Chem 2010; 285: 3840–3849.
Ponting C, Schultz J, Bork P . SPRY domains in ryanodine receptors (Ca(2+)-release channels). Trends Biochem Sci 1997; 22: 193–194.
Rhodes DA, de Bono B, Trowsdale J . Relationship between SPRY and B30.2 protein domains. Evolution of a component of immune defence? Immunology 2005; 116: 411–417.
Woo JS, Suh HY, Park SY, Oh BH . Structural basis for protein recognition by B30.2/SPRY domains. Mol Cell 2006; 24: 967–976.
Sells SF, Wood DP Jr., Joshi-Barve SS, Muthukumar S, Jacob RJ, Crist SA et al. Commonality of the gene programs induced by effectors of apoptosis in androgen-dependent and -independent prostate cells. Cell Growth Differ 1994; 5: 457–466.
El-Guendy N, Rangnekar VM . Apoptosis by Par-4 in cancer and neurodegenerative diseases. Exp Cell Res 2003; 283: 51–66.
El-Guendy N, Zhao Y, Gurumurthy S, Burikhanov R, Rangnekar VM . Identification of a unique core domain of par-4 sufficient for selective apoptosis induction in cancer cells. Mol Cell Biol 2003; 23: 5516–5525.
Chakraborty M, Qiu SG, Vasudevan KM, Rangnekar VM . Par-4 drives trafficking and activation of Fas and Fasl to induce prostate cancer cell apoptosis and tumor regression. Cancer Res 2001; 61: 7255–7263.
Nalca A, Qiu SG, El-Guendy N, Krishnan S, Rangnekar VM . Oncogenic Ras sensitizes cells to apoptosis by Par-4. J Biol Chem 1999; 274: 29976–29983.
Diaz-Meco MT, Lallena MJ, Monjas A, Frutos S, Moscat J . Inactivation of the inhibitory kappaB protein kinase/nuclear factor kappaB pathway by Par-4 expression potentiates tumor necrosis factor alpha-induced apoptosis. J Biol Chem 1999; 274: 19606–19612.
Gurumurthy S, Goswami A, Vasudevan KM, Rangnekar VM . Phosphorylation of Par-4 by protein kinase A is critical for apoptosis. Mol Cell Biol 2005; 25: 1146–1161.
Goswami A, Burikhanov R, de Thonel A, Fujita N, Goswami M, Zhao Y et al. Binding and phosphorylation of par-4 by akt is essential for cancer cell survival. Mol Cell 2005; 20: 33–44.
Ranganathan P, Rangnekar VM . Regulation of cancer cell survival by Par-4. Ann N Y Acad Sci 2005; 1059: 76–85.
Moreno-Bueno G, Fernandez-Marcos PJ, Collado M, Tendero MJ, Rodriguez-Pinilla SM, Garcia-Cao I et al. Inactivation of the candidate tumor suppressor par-4 in endometrial cancer. Cancer Res 2007; 67: 1927–1934.
Garcia-Cao I, Lafuente MJ, Criado LM, Diaz-Meco MT, Serrano M, Moscat J . Genetic inactivation of Par4 results in hyperactivation of NF-kappaB and impairment of JNK and p38. EMBO Rep 2003; 4: 307–312.
Zhao Y, Burikhanov R, Qiu S, Lele SM, Jennings CD, Bondada S et al. Cancer resistance in transgenic mice expressing the SAC module of Par-4. Cancer Res 2007; 67: 9276–9285.
Alvarez JV, Pan TC, Ruth J, Feng Y, Zhou A, Pant D et al. Par-4 downregulation promotes breast cancer recurrence by preventing multinucleation following targeted therapy. Cancer Cell 2013; 24: 30–44.
Wu C, Daniels RW, DiAntonio A . DFsn collaborates with Highwire to down-regulate the Wallenda/DLK kinase and restrain synaptic terminal growth. Neural Dev 2007; 2: 16.
Styhler S, Nakamura A, Lasko P . VASA localization requires the SPRY-domain and SOCS-box containing protein, GUSTAVUS. Dev Cell 2002; 3: 865–876.
Craig AG, Bandyopadhyay P, Olivera BM . Post-translationally modified neuropeptides from Conus venoms. Eur J Biochem 1999; 264: 271–275.
Masters SL, Yao S, Willson TA, Zhang JG, Palmer KR, Smith BJ et al. The SPRY domain of SSB-2 adopts a novel fold that presents conserved Par-4-binding residues. Nat Struct Mol Biol 2006; 13: 77–84.
Filippakopoulos P, Low A, Sharpe TD, Uppenberg J, Yao S, Kuang Z et al. Structural basis for Par-4 recognition by the SPRY domain- and SOCS box-containing proteins SPSB1, SPSB2, and SPSB4. J Mol Biol 2010; 401: 389–402.
Chaudhry P, Singh M, Parent S, Asselin E . Prostate apoptosis response 4 (Par-4), a novel substrate of caspase-3 during apoptosis activation. Mol Cell Biol 2012; 32: 826–839.
Azmi AS, Wang Z, Burikhanov R, Rangnekar VM, Wang G, Chen J et al. Critical role of prostate apoptosis response-4 in determining the sensitivity of pancreatic cancer cells to small-molecule inhibitor-induced apoptosis. Mol Cancer Ther 2008; 7: 2884–2893.
Sahasrabuddhe AA, Dimri M, Bommi PV, Dimri GP . betaTrCP regulates BMI1 protein turnover via ubiquitination and degradation. Cell Cycle 2011; 10: 1322–1330.
Acknowledgements
We thank Dr. Joon-Young Ahn for his assistance with the characterization of the anti-Fbxo45 polyclonal antibody. This work was funded by NIH grants R01 DE119249 and R01 CA136905 to KSJE-J and R01 CA140806 to MSL
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Edited by A Peschiaroli
Supplementary Information accompanies this paper on Cell Death and Differentiation website
Supplementary information
Rights and permissions
About this article
Cite this article
Chen, X., Sahasrabuddhe, A., Szankasi, P. et al. Fbxo45-mediated degradation of the tumor-suppressor Par-4 regulates cancer cell survival. Cell Death Differ 21, 1535–1545 (2014). https://doi.org/10.1038/cdd.2014.92
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/cdd.2014.92
This article is cited by
-
Fbxo45 facilitates the malignant progression of breast cancer by targeting Bim for ubiquitination and degradation
BMC Cancer (2024)
-
Fbxo45 promotes the malignant development of esophageal squamous cell carcinoma by targeting GGNBP2 for ubiquitination and degradation
Oncogene (2022)
-
Fbxo45 facilitates pancreatic carcinoma progression by targeting USP49 for ubiquitination and degradation
Cell Death & Disease (2022)
-
CACNA1C-AS2 inhibits cell proliferation and suppresses cell migration and invasion via targeting FBXO45 and PI3K/AKT/mTOR pathways in glioma
Apoptosis (2022)
-
Systematic analysis of the expression and prognosis relevance of FBXO family reveals the significance of FBXO1 in human breast cancer
Cancer Cell International (2021)