Abstract
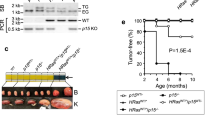
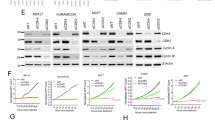
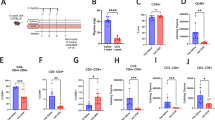
Cell-cycle inhibitors of the Ink4 and Cip/Kip families are involved in cellular senescence and tumor suppression. These inhibitors are individually dispensable for the cell cycle and inactivation of specific family members results in increased proliferation and enhanced susceptibility to tumor development. We have now analyzed the consequences of eliminating a substantial part of the cell-cycle inhibitory activity in the cell by generating a mouse model, which combines the absence of both p21Cip1 and p27Kip1 proteins with the endogenous expression of a Cdk4 R24C mutant insensitive to Ink4 inhibitors. Pairwise combination of Cdk4 R24C, p21-null and p27-null alleles results in frequent hyperplasias and tumors, mainly in cells of endocrine origin such as pituitary cells and in mesenchymal tissues. Interestingly, complete abrogation of p21Cip1 and p27Kip1 in Cdk4 R24C mutant mice results in a different phenotype characterized by perinatal death accompanied by general hypoplasia in most tissues. This phenotype correlates with increased replicative stress in developing tissues such as the nervous system and subsequent apoptotic cell death. Partial inhibition of Cdk4/6 rescues replicative stress signaling as well as p53 induction in the absence of cell-cycle inhibitors. We conclude that one of the major physiological activities of cell-cycle inhibitors is to prevent replicative stress during development.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
Abbreviations
- AC3:
-
active caspase 3
- Cdk:
-
cyclin-dependent kinase
- CKI:
-
Cdk inhibitor
- E:
-
embryonic day
- P:
-
postnatal day
- R:
-
R24C mutation in Cdk4
- Rb:
-
retinoblastoma
- pRb:
-
retinoblastoma protein
References
Sherr CJ . The Pezcoller lecture: cancer cell cycles revisited. Cancer Res 2000; 60: 3689–3695.
Malumbres M, Barbacid M . To cycle or not to cycle: a critical decision in cancer. Nat Rev Cancer 2001; 1: 222–231.
Malumbres M, Barbacid M . Cell cycle, CDKs and cancer: a changing paradigm. Nat Rev Cancer 2009; 9: 153–166.
Malumbres M, Barbacid M . Mammalian cyclin-dependent kinases. Trends Biochem Sci 2005; 30: 630–641.
Sherr CJ, Roberts JM . CDK inhibitors: positive and negative regulators of G1-phase progression. Genes Dev 1999; 13: 1501–1512.
Ruas M, Peters G . The p16INK4a/CDKN2A tumor suppressor and its relatives. Biochim Biophys Acta 1998; 1378: F115–F177.
Martin-Caballero J, Flores JM, Garcia-Palencia P, Serrano M . Tumor susceptibility of p21(Waf1/Cip1)-deficient mice. Cancer Res 2001; 61: 6234–6238.
Nakayama K, Ishida N, Shirane M, Inomata A, Inoue T, Shishido N et al. Mice lacking p27(Kip1) display increased body size, multiple organ hyperplasia, retinal dysplasia, and pituitary tumors. Cell 1996; 85: 707–720.
Fero ML, Rivkin M, Tasch M, Porter P, Carow CE, Firpo E et al. A syndrome of multiorgan hyperplasia with features of gigantism, tumorigenesis, and female sterility in p27(Kip1)-deficient mice. Cell 1996; 85: 733–744.
Kiyokawa H, Kineman RD, Manova-Todorova KO, Soares VC, Hoffman ES, Ono M et al. Enhanced growth of mice lacking the cyclin-dependent kinase inhibitor function of p27(Kip1). Cell 1996; 85: 721–732.
Latres E, Malumbres M, Sotillo R, Martín J, Ortega S, Martín-Caballero J et al. Limited overlapping roles of P15(INK4b) and P18(INK4c) cell cycle inhibitors in proliferation and tumorigenesis. EMBO J 2000; 19: 3496–3506.
Sharpless NE, Bardeesy N, Lee KH, Carrasco D, Castrillon DH, Aguirre AJ et al. Loss of p16Ink4a with retention of p19Arf predisposes mice to tumorigenesis. Nature 2001; 413: 86–91.
Krimpenfort P, Quon KC, Mooi WJ, Loonstra A, Berns A . Loss of p16Ink4a confers susceptibility to metastatic melanoma in mice. Nature 2001; 413: 83–86.
Krimpenfort P, Ijpenberg A, Song JY, van der Valk M, Nawijn M, Zevenhoven J et al. p15Ink4b is a critical tumour suppressor in the absence of p16Ink4a. Nature 2007; 448: 943–946.
Franklin DS, Godfrey VL, Lee H, Kovalev GI, Schoonhoven R, Chen-Kiang S et al. CDK inhibitors p18(INK4c) and p27(Kip1) mediate two separate pathways to collaboratively suppress pituitary tumorigenesis. Genes Dev 1998; 12: 2899–2911.
Ramsey MR, Krishnamurthy J, Pei XH, Torrice C, Lin W, Carrasco DR et al. Expression of p16Ink4a compensates for p18Ink4c loss in cyclin-dependent kinase 4/6-dependent tumors and tissues. Cancer Res 2007; 67: 4732–4741.
Martin-Caballero J, Flores JM, Garcia-Palencia P, Collado M, Serrano M . Different cooperating effect of p21 or p27 deficiency in combination with INK4a/ARF deletion in mice. Oncogene 2004; 23: 8231–8237.
Wolfel T, Hauer M, Schneider J, Serrano M, Wölfel C, Klehmann-Hieb E et al. A p16INK4a-insensitive CDK4 mutant targeted by cytolytic T lymphocytes in a human melanoma. Science 1995; 269: 1281–1284.
Sotillo R, Dubus P, Martin J, de la Cueva E, Ortega S, Malumbres M et al. Wide spectrum of tumors in knock-in mice carrying a Cdk4 protein insensitive to INK4 inhibitors. EMBO J 2001; 20: 6637–6647.
Sotillo R, Garcia JF, Ortega S, Martin J, Dubus P, Barbacid M et al. Invasive melanoma in Cdk4-targeted mice. Proc Natl Acad Sci USA 2001; 98: 13312–13317.
Sotillo R, Renner O, Dubus P, Ruiz-Cabello J, Martín-Caballero J, Barbacid M et al. Cooperation between Cdk4 and p27kip1 in tumor development: a preclinical model to evaluate cell cycle inhibitors with therapeutic activity. Cancer Res 2005; 65: 3846–3852.
Quereda V, Martinalbo J, Dubus P, Carnero A, Malumbres M . Genetic cooperation between p21Cip1 and INK4 inhibitors in cellular senescence and tumor suppression. Oncogene 2007; 26: 7665–7674.
Garcia-Fernandez RA, Garcia-Palencia P, Sanchez MA, Gil-Gómez G, Sánchez B, Rollán E et al. Combined loss of p21(waf1/cip1) and p27(kip1) enhances tumorigenesis in mice. Lab Invest 2011; 91: 1634–1642.
Quereda V, Malumbres M . Cell cycle control of pituitary development and disease. J Mol Endocrinol 2009; 42: 75–86.
Lange C, Huttner WB, Calegari F . Cdk4/cyclinD1 overexpression in neural stem cells shortens G1, delays neurogenesis, and promotes the generation and expansion of basal progenitors. Cell Stem Cell 2009; 5: 320–331.
Ryder EF, Snyder EY, Cepko CL . Establishment and characterization of multipotent neural cell lines using retrovirus vector-mediated oncogene transfer. J Neurobiol 1990; 21: 356–375.
Chu IM, Hengst L, Slingerland JM . The Cdk inhibitor p27 in human cancer: prognostic potential and relevance to anticancer therapy. Nat Rev Cancer 2008; 8: 253–267.
Coqueret O . New roles for p21 and p27 cell-cycle inhibitors: a function for each cell compartment? Trends Cell Biol 2003; 13: 65–70.
Gil J, Peters G . Regulation of the INK4b-ARF-INK4a tumour suppressor locus: all for one or one for all. Nat Rev Mol Cell Biol 2006; 7: 667–677.
Brugarolas J, Chandrasekaran C, Gordon JI, Beach D, Jacks T, Hannon GJ . Radiation-induced cell cycle arrest compromised by p21 deficiency. Nature 1995; 377: 552–557.
Franklin DS, Godfrey VL, O'Brien DA, Deng C, Xiong Y . Functional collaboration between different cyclin-dependent kinase inhibitors suppresses tumor growth with distinct tissue specificity. Mol Cell Biol 2000; 20: 6147–6158.
Rodriguez-Diez E, Quereda V, Bellutti F, Prchal-Murphy M, Partida D, Eguren M et al. Cdk4 and Cdk6 cooperate in counteracting the INK4 family of inhibitors during murine leukemogenesis. Blood 2014; 124: 2380–2390.
Eguren M, Porlan E, Manchado E, García-Higuera I, Cañamero M, Fariñas I et al. The APC/C cofactor Cdh1 prevents replicative stress and p53-dependent cell death in neural progenitors. Nat Commun 2013; 4: 2880.
Yurov YB, Vorsanova SG, Iourov IY . The DNA replication stress hypothesis of Alzheimer's disease. ScientificWorldJournal 2011; 11: 2602–2612.
Murga M, Bunting S, Montana MF, Soria R, Mulero F, Cañamero M et al. A mouse model of ATR-Seckel shows embryonic replicative stress and accelerated aging. Nat Genet 2009; 41: 891–898.
Farah MH, Olson JM, Sucic HB, Hume RI, Tapscott SJ, Turner DL . Generation of neurons by transient expression of neural bHLH proteins in mammalian cells. Development 2000; 127: 693–702.
Li X, Tang X, Jablonska B, Aguirre A, Gallo V, Luskin MB . p27(KIP1) regulates neurogenesis in the rostral migratory stream and olfactory bulb of the postnatal mouse. J Neurosci 2009; 29: 2902–2914.
Zheng YL, Li BS, Rudrabhatla P, Shukla V, Amin ND, Maric D et al. Phosphorylation of p27Kip1 at Thr187 by cyclin-dependent kinase 5 modulates neural stem cell differentiation. Mol Biol Cell 2010; 21: 3601–3614.
Pechnick RN, Zonis S, Wawrowsky K, Pourmorady J, Chesnokova V . p21Cip1 restricts neuronal proliferation in the subgranular zone of the dentate gyrus of the hippocampus. Proc Natl Acad Sci USA 2008; 105: 1358–1363.
Solomon DA, Kim JS, Jean W, Waldman T . Conspirators in a capital crime: co-deletion of p18INK4c and p16INK4a/p14ARF/p15INK4b in glioblastoma multiforme. Cancer Res 2008; 68: 8657–8660.
Bhatia B, Malik A, Fernandez LA, Kenney AM . p27(Kip1), a double-edged sword in Shh-mediated medulloblastoma: Tumor accelerator and suppressor. Cell Cycle 2010; 9: 4307–4314.
Murga M, Campaner S, Lopez-Contreras AJ, Toledo LI, Soria R, Montaña MF et al. Exploiting oncogene-induced replicative stress for the selective killing of Myc-driven tumors. Nat Struct Mol Biol 2011; 18: 1331–1335.
Qian X, Shen Q, Goderie SK, He W, Capela A, Davis AA et al. Timing of CNS cell generation: a programmed sequence of neuron and glial cell production from isolated murine cortical stem cells. Neuron 2000; 28: 69–80.
Trakala M, Fernandez-Miranda G, Perez de Castro I, Heeschen C, Malumbres M . Aurora B prevents delayed DNA replication and premature mitotic exit by repressing p21(Cip1). Cell Cycle 2013; 12: 1030–1041.
Garcia-Lavandeira M, Quereda V, Flores I, Saez C, Diaz-Rodriguez E, Japon MA et al. A GRFa2/Prop1/stem (GPS) cell niche in the pituitary. PLoS One 2009; 4: e4815.
Acknowledgements
We thank D Santamaría for critical comments on the manuscript. We thank Sally Temple and the members of her lab at the New York Neural Stem Cell Institute for guidelines in the work with neural progenitors. We also thank Beatriz Escobar and David Partida for excellent technical assistance, Sheila Rueda for colony mice management, and the members of the Confocal Microscopy and Comparative Pathology Units of the CNIO for their help. PD was supported by the Région Aquitaine and the Association pour la Recherche contre le Cancer (ARC). VQ was supported by fellowships from the Ministerio de Economía y Competitividad (MINECO). The Cell Division and Cancer group of the CNIO is funded by the MINECO (SAF2012-38215), Red Temática CellSYS (BFU2014-52125-REDT) and Red Consolider OncoBIO (SAF2014-57791-REDC), Comunidad de Madrid (OncoCycle Programme, S2010/BMD-2470), Worldwide Cancer Research (WCR #15-0278) and the EU MitoSys project (HEALTH-F5-2010-241548; European Union Seventh Framework Program).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Edited by A Villunger
Supplementary Information accompanies this paper on Cell Death and Differentiation website
Rights and permissions
About this article
Cite this article
Quereda, V., Porlan, E., Cañamero, M. et al. An essential role for Ink4 and Cip/Kip cell-cycle inhibitors in preventing replicative stress. Cell Death Differ 23, 430–441 (2016). https://doi.org/10.1038/cdd.2015.112
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/cdd.2015.112
This article is cited by
-
Cellular senescence or stemness: hypoxia flips the coin
Journal of Experimental & Clinical Cancer Research (2021)