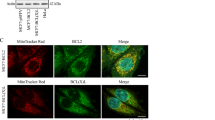
Abstract
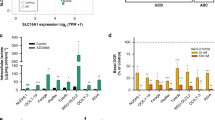
Diffuse large B-cell lymphomas (DLBCLs) are a highly heterogeneous group of tumors in which subsets share molecular features revealed by gene expression profiles and metabolic fingerprints. While B-cell receptor (BCR)-dependent DLBCLs are glycolytic, OxPhos-DLBCLs rely on mitochondrial energy transduction and nutrient utilization pathways that provide pro-survival benefits independent of BCR signaling. Integral to these metabolic distinctions is elevated mitochondrial electron transport chain (ETC) activity in OxPhos-DLBCLs compared with BCR-DLBCLs, which is linked to greater protein abundance of ETC components. To gain insights into molecular determinants of the selective increase in ETC activity and dependence on mitochondrial energy metabolism in OxPhos-DLBCLs, we examined the mitochondrial translation pathway in charge of the synthesis of mitochondrial DNA encoded ETC subunits. Quantitative mass spectrometry identified increased expression of mitochondrial translation factors in OxPhos-DLBCL as compared with the BCR subtype. Biochemical and functional assays indicate that the mitochondrial translation pathway is required for increased ETC activity and mitochondrial energy reserves in OxPhos-DLBCL. Importantly, molecular depletion of several mitochondrial translation proteins using RNA interference or pharmacological perturbation of the mitochondrial translation pathway with the FDA-approved inhibitor tigecycline (Tigecyl) is selectively toxic to OxPhos-DLBCL cell lines and primary tumors. These findings provide additional molecular insights into the metabolic characteristics of OxPhos-DLBCLs, and mark the mitochondrial translation pathway as a potential therapeutic target in these tumors.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
Abbreviations
- DLBCL:
-
diffuse large B-cell lymphoma
- BCR:
-
B-cell receptor
- OxPhos:
-
oxidative phosphorylation
- ETC:
-
electron transport chain
- mtDNA:
-
mitochondrial DNA
- COO:
-
cell-of-origin
- CCC:
-
consensus cluster classification
- FAO:
-
fatty acid oxidation
- iTRAQ:
-
isobaric tags for relative and absolute quantification
- DEEP SEQ:
-
deep efficient peptide sequencing and quantification
- TUFM:
-
Tu translation elongation factor mitochondrial
- GFM1:
-
G elongation factor mitochondria 1
- MRP:
-
mitochondrial ribosomal protein
- shRNA:
-
short hairpin RNA
- ROS:
-
reactive oxygen species
- NAC:
-
N-acetyl cysteine
- SRC:
-
spare respiratory capacity
- AML:
-
acute myeloid leukemia
References
Metallo CM, Vander Heiden MG . Understanding metabolic regulation and its influence on cell physiology. Mol Cell 2013; 49: 388–398.
Stanley IA, Ribeiro SM, Gimenez-Cassina A, Norberg E, Danial NN . Changing appetites: the adaptive advantages of fuel choice. Trends Cell Biol 2014; 24: 118–127.
Boroughs LK, DeBerardinis RJ . Metabolic pathways promoting cancer cell survival and growth. Nat Cell Biol 2015; 17: 351–359.
DeNicola GM, Cantley LC . Cancer's fuel choice: new flavors for a picky eater. Mol Cell 2015; 60: 514–523.
Lunt SY, Vander Heiden MG . Aerobic glycolysis: meeting the metabolic requirements of cell proliferation. Annu Rev Cell Dev Biol 2011; 27: 441–464.
Koppenol WH, Bounds PL, Dang CV . Otto Warburg's contributions to current concepts of cancer metabolism. Nat Rev Cancer 2011; 11: 325–337.
Caro P, Kishan AU, Norberg E, Stanley IA, Chapuy B, Ficarro SB et al. Metabolic signatures uncover distinct targets in molecular subsets of diffuse large B cell lymphoma. Cancer Cell 2012; 22: 547–560.
Mashimo T, Pichumani K, Vemireddy V, Hatanpaa KJ, Singh DK, Sirasanagandla S et al. Acetate is a bioenergetic substrate for human glioblastoma and brain metastases. Cell 2014; 159: 1603–1614.
Obre E, Rossignol R . Emerging concepts in bioenergetics and cancer research: Metabolic flexibility, coupling, symbiosis, switch, oxidative tumors, metabolic remodeling, signaling and bioenergetic therapy. Int J Biochem Cell Biol 2015; 59C: 167–181.
Schug ZT, Peck B, Jones DT, Zhang Q, Grosskurth S, Alam IS et al. Acetyl-CoA Synthetase 2 Promotes Acetate Utilization and Maintains Cancer Cell Growth under Metabolic Stress. Cancer Cell 2015; 27: 57–71.
Weinberg F, Hamanaka R, Wheaton WW, Weinberg S, Joseph J, Lopez M et al. Mitochondrial metabolism and ROS generation are essential for Kras-mediated tumorigenicity. Proc Natl Acad Sci USA 2010; 107: 8788–8793.
Yuneva MO, Fan TW, Allen TD, Higashi RM, Ferraris DV, Tsukamoto T et al. The metabolic profile of tumors depends on both the responsible genetic lesion and tissue type. Cell Metab 2012; 15: 157–170.
Wright G, Tan B, Rosenwald A, Hurt EH, Wiestner A, Staudt LM . A gene expression-based method to diagnose clinically distinct subgroups of diffuse large B cell lymphoma. Proc Natl Acad Sci USA 2003; 100: 9991–9996.
Monti S, Savage KJ, Kutok JL, Feuerhake F, Kurtin P, Mihm M et al. Molecular profiling of diffuse large B-cell lymphoma identifies robust subtypes including one characterized by host inflammatory response. Blood 2005; 105: 1851–1861.
Monti S, Chapuy B, Takeyama K, Rodig SJ, Hao Y, Yeda KT et al. Integrative analysis reveals an outcome-associated and targetable pattern of p53 and cell cycle deregulation in diffuse large B cell lymphoma. Cancer Cell 2012; 22: 359–372.
Chen L, Monti S, Juszczynski P, Ouyang J, Chapuy B, Neuberg D et al. SYK inhibition modulates distinct PI3K/AKT- dependent survival pathways and cholesterol biosynthesis in diffuse large B cell lymphomas. Cancer Cell 2013; 23: 826–838.
Pfeifer M, Grau M, Lenze D, Wenzel SS, Wolf A, Wollert-Wulf B et al. PTEN loss defines a PI3K/AKT pathway-dependent germinal center subtype of diffuse large B-cell lymphoma. Proc Natl Acad Sci USA 2013; 110: 12420–12425.
Gustafsson CM, Falkenberg M, Larsson NG . Maintenance and Expression of Mammalian Mitochondrial DNA. Annu Rev Biochem 2016; 85: 133–160.
Rebelo AP, Dillon LM, Moraes CT, Mitochondrial DNA . transcription regulation and nucleoid organization. J Inherit Metab Dis 2011; 34: 941–951.
Ott M, Amunts A, Brown A . Organization and Regulation of Mitochondrial Protein Synthesis. Annu Rev Biochem 2016; 85: 77–101.
Shahni R, Wedatilake Y, Cleary MA, Lindley KJ, Sibson KR, Rahman S . A distinct mitochondrial myopathy, lactic acidosis and sideroblastic anemia (MLASA) phenotype associates with YARS2 mutations. Am J Med Genet A 2013; 161: 2334–2338.
Baker MJ, Tatsuta T, Langer T . Quality control of mitochondrial proteostasis. Cold Spring Harb Perspect Biol 2011; 3: a00755.
Enriquez JA . Supramolecular organization of respiratory complexes. Annu Rev Physiol 2016; 78: 533–561.
Zhou F, Lu Y, Ficarro SB, Webber JT, Marto JA . Nanoflow low pressure high peak capacity single dimension LC-MS/MS platform for high-throughput, in-depth analysis of mammalian proteomes. Anal Chem 2012; 84: 5133–5139.
Zhou F, Lu Y, Ficarro SB, Adelmant G, Jiang W, Luckey CJ et al. Genome-scale proteome quantification by DEEP SEQ mass spectrometry. Nat Commun 2013; 4: 2171.
Alizadeh AA, Eisen MB, Davis RE, Ma C, Lossos IS, Rosenwald A et al. Distinct types of diffuse large B-cell lymphoma identified by gene expression profiling. Nature 2000; 403: 503–511.
Polo JM, Juszczynski P, Monti S, Cerchietti L, Ye K, Greally JM et al. Transcriptional signature with differential expression of BCL6 target genes accurately identifies BCL6-dependent diffuse large B cell lymphomas. Proc Natl Acad Sci USA 2007; 104: 3207–3212.
Bestwick ML, Shadel GS . Accessorizing the human mitochondrial transcription machinery. Trends Biochem Sci 2013; 38: 283–291.
Echevarria L, Clemente P, Hernandez-Sierra R, Gallardo ME, Fernandez-Moreno MA, Garesse R . Glutamyl-tRNAGln amidotransferase is essential for mammalian mitochondrial translation in vivo. Biochem J 2014; 460: 91–101.
Rorbach J, Richter R, Wessels HJ, Wydro M, Pekalski M, Farhoud M et al. The human mitochondrial ribosome recycling factor is essential for cell viability. Nucleic Acids Res 2008; 36: 5787–5799.
Jenner L, Starosta AL, Terry DS, Mikolajka A, Filonava L, Yusupov M et al. Structural basis for potent inhibitory activity of the antibiotic tigecycline during protein synthesis. Proc Natl Acad Sci USA 2013; 110: 3812–3816.
Muralidharan G, Micalizzi M, Speth J, Raible D, Troy S . Pharmacokinetics of tigecycline after single and multiple doses in healthy subjects. Antimicrob Agents Chemother 2005; 49: 220–229.
Wenzel R, Bate G, Kirkpatrick P . Tigecycline. Nat Rev Drug Discov 2005; 4: 809–810.
Skrtic M, Sriskanthadevan S, Jhas B, Gebbia M, Wang X, Wang Z et al. Inhibition of mitochondrial translation as a therapeutic strategy for human acute myeloid leukemia. Cancer Cell 2011; 20: 674–688.
Acin-Perez R, Bayona-Bafaluy MP, Fernandez-Silva P, Moreno-Loshuertos R, Perez-Martos A, Bruno C et al. Respiratory complex III is required to maintain complex I in mammalian mitochondria. Mol Cell 2004; 13: 805–815.
Diaz F, Fukui H, Garcia S, Moraes CT . Cytochrome c oxidase is required for the assembly/stability of respiratory complex I in mouse fibroblasts. Mol Cell Biol 2006; 26: 4872–4881.
Schagger H, de Coo R, Bauer MF, Hofmann S, Godinot C, Brandt U . Significance of respirasomes for the assembly/stability of human respiratory chain complex I. J Biol Chem 2004; 279: 36349–36353.
Maranzana E, Barbero G, Falasca AI, Lenaz G, Genova ML . Mitochondrial respiratory supercomplex association limits production of reactive oxygen species from complex I. Antioxid Redox Signal 2013; 19: 1469–1480.
Dranka BP, Hill BG, Darley-Usmar VM . Mitochondrial reserve capacity in endothelial cells: The impact of nitric oxide and reactive oxygen species. Free Radic Biol Med 2010; 48: 905–914.
Nicholls DG . Spare respiratory capacity, oxidative stress and excitotoxicity. Biochem Soc Trans 2009; 37: 1385–1388.
Orrenius S, Gogvadze V, Zhivotovsky B . Mitochondrial oxidative stress: implications for cell death. Annu Rev Pharmacol Toxicol 2007; 47: 143–183.
Schieber M, Chandel NS . ROS function in redox signaling and oxidative stress. Curr Biol 2014; 24: R453–R462.
Brand MD . Mitochondrial generation of superoxide and hydrogen peroxide as the source of mitochondrial redox signaling. Free Radic Biol Med 2016 (e-pub ahead of print 13 April 2016; doi:10.1016/j.freeradbiomed.2016.04.001).
Murphy MP . How mitochondria produce reactive oxygen species. Biochem J 2009; 417: 1–13.
Nagar H, Jung SB, Kwon SK, Park JB, Shong M, Song HJ et al. CRIF1 deficiency induces p66shc-mediated oxidative stress and endothelial activation. PLoS One 2014; 9: e98670.
Schedlbauer A, Kaminishi T, Ochoa-Lizarralde B, Dhimole N, Zhou S, Lopez-Alonso JP et al. Structural characterization of an alternative mode of tigecycline binding to the bacterial ribosome. Antimicrob Agents Chemother 2015; 59: 2849–2854.
Amunts A, Brown A, Toots J, Scheres SH, Ramakrishnan V . Ribosome. The structure of the human mitochondrial ribosome. Science 2015; 348: 95–98.
Scarpulla RC, Vega RB, Kelly DP . Transcriptional integration of mitochondrial biogenesis. Trends Endocrinol Metab 2012; 23: 459–466.
Sotgia F, Whitaker-Menezes D, Martinez-Outschoorn UE, Salem AF, Tsirigos A, Lamb R et al. Mitochondria "fuel" breast cancer metabolism: fifteen markers of mitochondrial biogenesis label epithelial cancer cells, but are excluded from adjacent stromal cells. Cell Cycle 2012; 11: 4390–4401.
Friedberg JW, Sharman J, Sweetenham J, Johnston PB, Vose JM, Lacasce A et al. Inhibition of Syk with fostamatinib disodium has significant clinical activity in non-Hodgkin lymphoma and chronic lymphocytic leukemia. Blood 2010; 115: 2578–2585.
Younes A, Thieblemont C, Morschhauser F, Flinn I, Friedberg JW, Amorim S et al. Combination of ibrutinib with rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone (R-CHOP) for treatment-naive patients with CD20-positive B-cell non-Hodgkin lymphoma: a non-randomised, phase 1b study. Lancet Oncol 2014; 15: 1019–1026.
Corazao-Rozas P, Guerreschi P, Jendoubi M, Andre F, Jonneaux A, Scalbert C et al. Mitochondrial oxidative stress is the Achille's heel of melanoma cells resistant to Braf-mutant inhibitor. Oncotarget 2013; 4: 1986–1998.
Viale A, Pettazzoni P, Lyssiotis CA, Ying H, Sanchez N, Marchesini M et al. Oncogene ablation-resistant pancreatic cancer cells depend on mitochondrial function. Nature 2014; 514: 628–632.
Bucaneve G, Micozzi A, Picardi M, Ballanti S, Cascavilla N, Salutari P et al. Results of a multicenter, controlled, randomized clinical trial evaluating the combination of piperacillin/tazobactam and tigecycline in high-risk hematologic patients with cancer with febrile neutropenia. J Clin Oncol 2014; 32: 1463–1471.
Chen L, Monti S, Juszczynski P, Daley J, Chen W, Witzig TE et al. SYK-dependent tonic B-cell receptor signaling is a rational treatment target in diffuse large B-cell lymphoma. Blood 2008; 111: 2230–2237.
Davis RE, Ngo VN, Lenz G, Tolar P, Young RM, Romesser PB et al. Chronic active B-cell-receptor signalling in diffuse large B-cell lymphoma. Nature 2010; 463: 88–92.
Askenazi M, Parikh JR, Marto JA . mzAPI: a new strategy for efficiently sharing mass spectrometry data. Nat Methods 2009; 6: 240–241.
Parikh JR, Askenazi M, Ficarro SB, Cashorali T, Webber JT, Blank NC et al. multiplierz: an extensible API based desktop environment for proteomics data analysis. BMC Bioinformatics 2009; 10: 364.
Jitkova Y, Gronda M, Hurren R, Wang X, Goard CA, Jhas B et al. A novel formulation of tigecycline has enhanced stability and sustained antibacterial and antileukemic activity. PLoS One 2014; 9: e95281.
Acin-Perez R, Fernandez-Silva P, Peleato ML, Perez-Martos A, Enriquez JA . Respiratory active mitochondrial supercomplexes. Mol Cell 2008; 32: 529–539.
Acknowledgements
We thank Elaura Patton, Meghan Tedoldi, and Heather Sun for technical assistance, Rebecca Acin-Pérez and José Antonio Enriquez for advice on mitochondrial supercomplexes, and Benjamin Szlyk for graphics. This work was supported by the US National Institutes of Health grants R21 CA178860 (NND and JAM), V Foundation for Cancer Research (NND and MAS), F31 CA171400 (IAS), the Swedish Society for Medical Research (SSMF), and The Malin and Lennart Philipson Foundation (EN). We also acknowledge generous support provided through the Dana-Farber Cancer Institute Strategic Research Initiative and CA188881 (JAM).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Edited by N Chandel
Supplementary Information accompanies this paper on Cell Death and Differentiation website
Supplementary information
Rights and permissions
About this article
Cite this article
Norberg, E., Lako, A., Chen, PH. et al. Differential contribution of the mitochondrial translation pathway to the survival of diffuse large B-cell lymphoma subsets. Cell Death Differ 24, 251–262 (2017). https://doi.org/10.1038/cdd.2016.116
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/cdd.2016.116
This article is cited by
-
SH3BP5-driven metabolic-immune crosstalk in DLBCL: a prognostic biomarker and therapeutic target for reshaping immunosuppressive microenvironment
Journal of Translational Medicine (2025)
-
MPC2 Overexpression Drives Mitochondrial Oxidative Phosphorylation and Promotes Progression in Diffuse Large B-Cell Lymphoma
Biochemical Genetics (2025)
-
Unraveling the roles and mechanisms of mitochondrial translation in normal and malignant hematopoiesis
Journal of Hematology & Oncology (2024)
-
Transcriptomic analysis delineates potential regulatory network as therapeutic alternatives in chronic myeloid leukemia
Egyptian Journal of Medical Human Genetics (2024)
-
Mitochondria in tumor immune surveillance and tumor therapies targeting mitochondria
Cellular Oncology (2024)