Abstract
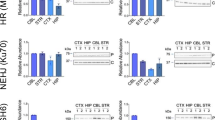
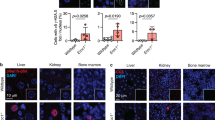
We observed that the transient induction of mtDNA double strand breaks (DSBs) in cultured cells led to activation of cell cycle arrest proteins (p21/p53 pathway) and decreased cell growth, mediated through reactive oxygen species (ROS). To investigate this process in vivo we developed a mouse model where we could transiently induce mtDNA DSBs ubiquitously. This transient mtDNA damage in mice caused an accelerated aging phenotype, preferentially affecting proliferating tissues. One of the earliest phenotypes was accelerated thymus shrinkage by apoptosis and differentiation into adipose tissue, mimicking age-related thymic involution. This phenotype was accompanied by increased ROS and activation of cell cycle arrest proteins. Treatment with antioxidants improved the phenotype but the knocking out of p21 or p53 did not. Our results demonstrate that transient mtDNA DSBs can accelerate aging of certain tissues by increasing ROS. Surprisingly, this mtDNA DSB-associated senescence phenotype does not require p21/p53, even if this pathway is activated in the process.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
Abbreviations
- mtDNA:
-
mitochondrial DNA
- DSBs:
-
double strand breaks
- ROS:
-
reactive oxygen species
- OXPHOS:
-
oxidative phosphorylation
- COX8:
-
cytochrome c oxidase subunit VIII
- Cdkn1a :
-
cyclin-dependent kinase Inhibitor 1 A (p21/Cip1)
- MDM2:
-
Mouse double minute 2
- NAC:
-
N-acetyl-cysteine
- TUNEL:
-
terminal deoxynucleotidyl transferase dUTP nick end labeling
- DOX:
-
doxycycline
- DEXA:
-
dual-energy X-ray absorptiometry
- DN:
-
double negative, CD4−/CD8−
- DP:
-
double positive CD4+/CD8+
- PGC:
-
peroxisome proliferator activated receptor gamma coactivators
- PPARγ:
-
peroxisome proliferator activated receptor gamma
- ADRP:
-
adipose differentiation related protein
- SystemicIndmito-PstI:
-
systemic-inducible mitochondrial-targeted PstI
References
Johnson FB, Sinclair DA, Guarente L . Molecular biology of aging. Cell 1999; 96: 291–302.
Sedelnikova OA, Horikawa I, Zimonjic DB, Popescu NC, Bonner WM, Barrett JC . Senescing human cells and ageing mice accumulate DNA lesions with unrepairable double-strand breaks. Nat Cell Biol 2004; 6: 168–170.
Vogel H, Lim DS, Karsenty G, Finegold M, Hasty P . Deletion of Ku86 causes early onset of senescence in mice. Proc Natl Acad Sci USA 1999; 96: 10770–10775.
Murga M, Bunting S, Montana MF, Soria R, Mulero F, Canamero M et al. A mouse model of ATR-Seckel shows embryonic replicative stress and accelerated aging. Nat Genet 2009; 41: 891–898.
Inomata K, Aoto T, Binh NT, Okamoto N, Tanimura S, Wakayama T et al. Genotoxic stress abrogates renewal of melanocyte stem cells by triggering their differentiation. Cell 2009; 137: 1088–1099.
Ruzankina Y, Schoppy DW, Asare A, Clark CE, Vonderheide RH, Brown EJ . Tissue regenerative delays and synthetic lethality in adult mice after combined deletion of Atr and Trp53. Nat Genet 2009; 41: 1144–1149.
Ruzankina Y, Pinzon-Guzman C, Asare A, Ong T, Pontano L, Cotsarelis G et al. Deletion of the developmentally essential gene ATR in adult mice leads to age-related phenotypes and stem cell loss. Cell Stem Cell 2007; 1: 113–126.
Rodier F, Campisi J, Bhaumik D . Two faces of p53: aging and tumor suppression. Nucleic Acids Res 2007; 35: 7475–7484.
Renault VM, Thekkat PU, Hoang KL, White JL, Brady CA, Kenzelmann Broz D et al. The pro-longevity gene FoxO3 is a direct target of the p53 tumor suppressor. Oncogene 2011; 30: 3207–3221.
Gudkov AV, Gurova KV, Komarova EA . Inflammation and p53: a tale of two stresses. Genes Cancer 2011; 2: 503–516.
Feng Z, Lin M, Wu R . The regulation of aging and longevity: a new and complex role of p53. Genes Cancer 2011; 2: 443–452.
Balaban RS, Nemoto S, Finkel T . Mitochondria, oxidants, and aging. Cell 2005; 120: 483–495.
Schumacher B, van der Pluijm I, Moorhouse MJ, Kosteas T, Robinson AR, Suh Y et al. Delayed and accelerated aging share common longevity assurance mechanisms. PLoS Genet 2008; 4: e1000161.
McCarroll SA, Murphy CT, Zou S, Pletcher SD, Chin CS, Jan YN et al. Comparing genomic expression patterns across species identifies shared transcriptional profile in aging. Nat Genet 2004; 36: 197–204.
Zahn JM, Sonu R, Vogel H, Crane E, Mazan-Mamczarz K, Rabkin R et al. Transcriptional profiling of aging in human muscle reveals a common aging signature. PLoS Genet 2006; 2: e115.
Anderson S, Bankier AT, Barrell BG, de Bruijn MH, Coulson AR, Drouin J et al. Sequence and organization of the human mitochondrial genome. Nature 1981; 290: 457–465.
Harman D . Aging: a theory based on free radical and radiation chemistry. J gerontol 1956; 11: 298–300.
Wallace DC . Mitochondrial DNA mutations in disease and aging. Environ mol mutagen 2010; 51: 440–450.
Vermulst M, Bielas JH, Kujoth GC, Ladiges WC, Rabinovitch PS, Prolla TA et al. Mitochondrial point mutations do not limit the natural lifespan of mice. Nat Genet 2007; 39: 540–543.
Vermulst M, Wanagat J, Kujoth GC, Bielas JH, Rabinovitch PS, Prolla TA et al. DNA deletions and clonal mutations drive premature aging in mitochondrial mutator mice. Nat Genet 2008; 40: 392–394.
Schaar CE, Dues DJ, Spielbauer KK, Machiela E, Cooper JF, Senchuk M et al. Mitochondrial and cytoplasmic ROS have opposing effects on lifespan. PLoS genet 2015; 11: e1004972.
Van Raamsdonk JM, Hekimi S . Deletion of the mitochondrial superoxide dismutase sod-2 extends lifespan in Caenorhabditis elegans. PLoS genet 2009; 5: e1000361.
Bacman SR, Williams SL, Moraes CT . Intra- and inter-molecular recombination of mitochondrial DNA after in vivo induction of multiple double-strand breaks. Nucleic acids res 2009; 37: 4218–4226.
Zeke A, Misheva M, Remenyi A, Bogoyevitch MA . JNK signaling: regulation and functions based on complex protein-protein partnerships. Microbiol mol biol rev 2016; 80: 793–835.
Munshi A, Ramesh R . Mitogen-activated protein kinases and their role in radiation response. Genes cancer 2013; 4: 401–408.
Lee SB, Bae IH, Bae YS, Um HD . Link between mitochondria and NADPH oxidase 1 isozyme for the sustained production of reactive oxygen species and cell death. J Biol Chem 2006; 281: 36228–36235.
Belteki G, Haigh J, Kabacs N, Haigh K, Sison K, Costantini F et al. Conditional and inducible transgene expression in mice through the combinatorial use of Cre-mediated recombination and tetracycline induction. Nucleic Acids Res 2005; 33: e51.
Fukui H, Moraes CT . Mechanisms of formation and accumulation of mitochondrial DNA deletions in aging neurons. Hum mol genet 2009; 18: 1028–1036.
Awong G, Zuniga-Pflucker JC . Thymus-bound: the many features of T cell progenitors. Front Biosci (Schol Ed) 2011; 3: 961–969.
Marinova TT . Epithelial framework reorganization during human thymus involution. Gerontology 2005; 51: 14–18.
Puigserver P, Wu ZD, Park CW, Graves R, Wright M, Spiegelman BM . A cold-inducible coactivator of nuclear receptors linked to adaptive thermogenesis. Cell 1998; 92: 829–839.
Rosen ED, Sarraf P, Troy AE, Bradwin G, Moore K, Milstone DS et al. PPAR gamma is required for the differentiation of adipose tissue in vivo and in vitro. Mol Cell 1999; 4: 611–617.
Brun RP, Tontonoz P, Forman BM, Ellis R, Chen J, Evans RM et al. Differential activation of adipogenesis by multiple PPAR isoforms. Gene Dev 1996; 10: 974–984.
Lozano G, Levine AJ . Tissue-specific expression of P53 in transgenic mice is regulated by intron sequences. Mol Carcinogen 1991; 4: 3–9.
Tyner SD, Venkatachalam S, Choi J, Jones S, Ghebranious N, Igelmann H et al. p53 mutant mice that display early ageing-associated phenotypes. Nature 2002; 415: 45–53.
Liu DP, Ou LD, Clemenson GD, Chao C, Lutske ME, Zambetti GP et al. Puma is required for p53-induced depletion of adult stem cells. Nat Cell Biol 2010; 12: 993–998.
Lavin MF, Gueven N . The complexity of p53 stabilization and activation. Cell Death Differ 2006; 13: 941–950.
Ahlqvist KJ, Hamalainen RH, Yatsuga S, Uutela M, Terzioglu M, Gotz A et al. Somatic progenitor cell vulnerability to mitochondrial dna mutagenesis underlies progeroid phenotypes in polg mutator mice. Cell Metab 2012; 15: 100–109.
Deng C, Zhang P, Harper JW, Elledge SJ, Leder P . Mice lacking p21CIP1/WAF1 undergo normal development, but are defective in G1 checkpoint control. Cell 1995; 82: 675–684.
Jacks T, Remington L, Williams BO, Schmitt EM, Halachmi S, Bronson RT et al. Tumor spectrum analysis in p53-mutant mice. Curr biol 1994; 4: 1–7.
Coffey G, Campbell C . An alternate form of Ku80 is required for DNA end-binding activity in mammalian mitochondria. Nucleic Acids Res 2000; 28: 3793–3800.
Lakshmipathy U, Campbell C . Mitochondrial DNA ligase III function is independent of xrcc1. Nucleic Acids Res 2000; 28: 3880–3886.
Lakshmipathy U, Campbell C . The human DNA ligase III gene encodes nuclear and mitochondrial proteins. Mol Cell Biol 1999; 19: 3869–3876.
Eaton JS, Lin ZP, Sartorelli AC, Bonawitz ND, Shadel GS . Ataxia-telangiectasia mutated kinase regulates ribonucleotide reductase and mitochondrial homeostasis. J Clin Invest 2007; 117: 2723–2734.
Valentin-Vega YA, MacLean KH, Tait-Mulder J, Milasta S, Steeves M, Dorsey FC et al. Mitochondrial dysfunction in ataxia-telangiectasia. Blood 2012; 119: 1490–1500.
Sage JM, Gildemeister OS, Knight KL . Discovery of a novel function for human Rad51 maintenance of the mitochondrial genome. J Biol Chem 2010; 285: 18984–18990.
Bayona-Bafaluy MP, Blits B, Battersby BJ, Shoubridge EA, Moraes CT . Rapid directional shift of mitochondrial DNA heteroplasmy in animal tissues by a mitochondrially targeted restriction endonuclease. Proc Natl Acad Sci USA 2005; 102: 14392–14397.
Wang X, Pickrell AM, Rossi SG, Pinto M, Dillon LM, Hida A et al. Transient systemic mtDNA damage leads to muscle wasting by reducing the satellite cells pool. Hum mol genet 2013; 22: 3976–3986.
Guo Z, Kozlov S, Lavin MF, Person MD, Paull TT . ATM activation by oxidative stress. Science 2010; 330: 517–521.
Hartman P, Ponder R, Lo HH, Ishii N . Mitochondrial oxidative stress can lead to nuclear hypermutability. Mech Ageing Dev 2004; 125: 417–420.
Lauritzen KH, Cheng C, Wiksen H, Bergersen LH, Klungland A . Mitochondrial DNA toxicity compromises mitochondrial dynamics and induces hippocampal antioxidant defenses. DNA Repair 2011; 10: 639–653.
Pohjoismaki JL, Williams SL, Boettger T, Goffart S, Kim J, Suomalainen A et al. Overexpression of Twinkle-helicase protects cardiomyocytes from genotoxic stress caused by reactive oxygen species. Proc Natl Acad Sci USA 2013; 110: 19408–19413.
Bacman SR, Williams SL, Moraes CT . Intra- and inter-molecular recombination of mitochondrial DNA after in vivo induction of multiple double-strand breaks. Nucleic Acids Res 2009; 37: 4218–4226.
Pickrell AM, Fukui H, Wang X, Pinto M, Moraes CT . The striatum is highly susceptible to mitochondrial oxidative phosphorylation dysfunctions. J neurosci 2011; 31: 9895–9904.
Srivastava S, Moraes CT . Manipulating mitochondrial DNA heteroplasmy by a mitochondrially targeted restriction endonuclease. Hum mol genet 2001; 10: 3093–3099.
Schmittgen TD, Livak KJ . Analyzing real-time PCR data by the comparative C-T method. Nat Protoc 2008; 3: 1101–1108.
Bayer AL, Yu AX, Malek TR . Function of the IL-2R for thymic and peripheral CD4(+)CD25(+) Foxp3(+) T regulatory cells. J Immunol 2007; 178: 4062–4071.
Acknowledgements
We would like to thank Dr. Wayne E. Balkan from the University of Miami for the access to and the use of the DEXA scan; Dr. Norman H. Altman VMD for consultation on thymus pathology, Dr. Ying Wang, and Dr. Ge Tao from the University of Miami for antibodies and technical assistance. We also thank the University of Miami Comparative Pathology Laboratory for the blood work analysis. This work was supported primarily by the US National Institutes of Health Grant 1R01AG036871. The following grants also helped support this work: NIH 1R01NS079965, 5R01EY010804; the Muscular Dystrophy Association and the United Mitochondrial Disease Foundation. We acknowledge support from the NEI center grant P30-EY014801 from the National Institutes of Health (NIH).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Edited by N Chandel
Supplementary Information accompanies this paper on Cell Death and Differentiation website
Rights and permissions
About this article
Cite this article
Pinto, M., Pickrell, A., Wang, X. et al. Transient mitochondrial DNA double strand breaks in mice cause accelerated aging phenotypes in a ROS-dependent but p53/p21-independent manner. Cell Death Differ 24, 288–299 (2017). https://doi.org/10.1038/cdd.2016.123
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/cdd.2016.123
This article is cited by
-
Changes in transposable elements expression in male and female mice liver throughout aging
GeroScience (2026)
-
Development and validation of AI-based automatic segmentation and measurement of thymus on chest CT scans
BMC Medical Imaging (2025)
-
Divergent immediate and delayed effects of juvenile exposure to doxorubicin on the thymus in C57BL/6 mice
Scientific Reports (2025)
-
Impaired Complex I dysregulates neural/glial precursors and corpus callosum development revealing postnatal defects in Leigh syndrome mice
EMBO Molecular Medicine (2025)
-
Canagliflozin improves high-salt-induced aortic arteriosclerosis and premature aging in dahl salt-sensitive rats through the SIRT6/HIF-1 α signaling pathway
Molecular and Cellular Biochemistry (2025)