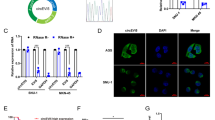
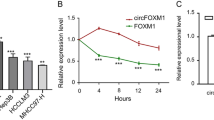
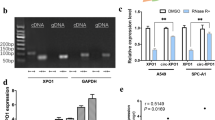
Abstract
Circular RNAs are a class of non-coding RNAs that are receiving extensive attention. Despite reports showing circular RNAs acting as microRNA sponges, the biological functions of circular RNAs remain largely unknown. We show that in patient tumor samples and in a panel of cancer cells, circ-Foxo3 was minimally expressed. Interestingly, during cancer cell apoptosis, the expression of circ-Foxo3 was found to be significantly increased. We found that silencing endogenous circ-Foxo3 enhanced cell viability, whereas ectopic expression of circ-Foxo3 triggered stress-induced apoptosis and inhibited the growth of tumor xenografts. Also, expression of circ-Foxo3 increased Foxo3 protein levels but repressed p53 levels. By binding to both, circ-Foxo3 promoted MDM2-induced p53 ubiquitination and subsequent degradation, resulting in an overall decrease of p53. With low binding affinity to Foxo3 protein, circ-Foxo3 prevented MDM2 from inducing Foxo3 ubiquitination and degradation, resulting in increased levels of Foxo3 protein. As a result, cell apoptosis was induced by upregulation of the Foxo3 downstream target PUMA.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Jeck WR, Sharpless NE . Detecting and characterizing circular RNAs. Nat Biotechnol 2014; 32: 453–461.
Haimovich G, Medina DA, Causse SZ, Garber M, Millan-Zambrano G, Barkai O et al. Gene expression is circular: factors for mRNA degradation also foster mRNA synthesis. Cell 2013; 153: 1000–1011.
Wilusz JE, Sharp PA . Molecular biology. A circuitous route to noncoding RNA. Science 2013; 340: 440–441.
Westholm JO, Miura P, Olson S, Shenker S, Joseph B, Sanfilippo P et al. Genome-wide analysis of drosophila circular RNAs reveals their structural and sequence properties and age-dependent neural accumulation. Cell Rep 2014; 9: 1966–1980.
Zhang XO, Wang HB, Zhang Y, Lu X, Chen LL, Yang L . Complementary sequence-mediated exon circularization. Cell 2014; 159: 134–147.
Zhang Y, Zhang XO, Chen T, Xiang JF, Yin QF, Xing YH et al. Circular intronic long noncoding RNAs. Mol Cell 2013; 51: 792–806.
Hansen TB, Jensen TI, Clausen BH, Bramsen JB, Finsen B, Damgaard CK et al. Natural RNA circles function as efficient microRNA sponges. Nature 2013; 495: 384–388.
Yang W, Du WW, Li X, Yee AJ, Yang BB . Foxo3 activity promoted by non-coding effects of circular RNA and Foxo3 pseudogene in the inhibition of tumor growth and angiogenesis. Oncogene 2016; 35: 3919–3931.
Li Z, Huang C, Bao C, Chen L, Lin M, Wang X et al. Exon-intron circular RNAs regulate transcription in the nucleus. Nat Struct Mol Biol 2015; 22: 256–264.
Du WW, Yang W, Chen Y, Wu ZK, Foster FS, Yang Z et al. Foxo3 circular RNA promotes cardiac senescence by modulating multiple factors associated with stress and senescence responses. Eur Heart J 2016 (in press).
Du WW, Yang W, Liu E, Yang Z, Dhaliwal P, Yang BB . Foxo3 circular RNA retards cell cycle progression via forming ternary complexes with p21 and CDK2. Nucleic Acids Res 2016; 44: 2846–2858.
Elenbaas B, Dobbelstein M, Roth J, Shenk T, Levine AJ . The MDM2 oncoprotein binds specifically to RNA through its RING finger domain. Mol Med 1996; 2: 439–451.
Yoshida Y, Izumi H, Torigoe T, Ishiguchi H, Yoshida T, Itoh H et al. Binding of RNA to p53 regulates its oligomerization and DNA-binding activity. Oncogene 2004; 23: 4371–4379.
Myatt SS, Lam EW . The emerging roles of forkhead box (Fox) proteins in cancer. Nat Rev Cancer 2007; 7: 847–859.
Cho EC, Kuo ML, Liu X, Yang L, Hsieh YC, Wang J et al. Tumor suppressor FOXO3 regulates ribonucleotide reductase subunit RRM2B and impacts on survival of cancer patients. Oncotarget 2014; 5: 4834–4844.
Anderson MJ, Viars CS, Czekay S, Cavenee WK, Arden KC . Cloning and characterization of three human forkhead genes that comprise an FKHR-like gene subfamily. Genomics 1998; 47: 187–199.
Hofacker IL, Stadler PF . Memory efficient folding algorithms for circular RNA secondary structures. Bioinformatics 2006; 22: 1172–1176.
Zuker M . Mfold web server for nucleic acid folding and hybridization prediction. Nucleic Acids Res 2003; 31: 3406–3415.
Popenda M, Szachniuk M, Antczak M, Purzycka KJ, Lukasiak P, Bartol N et al. Automated 3D structure composition for large RNAs. Nucleic Acids Res 2012; 40: e112.
Surget S, Khoury MP . Bourdon JC. Uncovering the role of p53 splice variants in human malignancy: a clinical perspective. Onco Targets Ther 2013; 7: 57–68.
Bullock AN, Henckel J, DeDecker BS, Johnson CM, Nikolova PV, Proctor MR et al. Thermodynamic stability of wild-type and mutant p53 core domain. Proc Natl Acad Sci USA 1997; 94: 14338–14342.
Ekoff M, Kaufmann T, Engstrom M, Motoyama N, Villunger A, Jonsson JI et al. The BH3-only protein Puma plays an essential role in cytokine deprivation induced apoptosis of mast cells. Blood 2007; 110: 3209–3217.
Haupt Y, Maya R, Kazaz A, Oren M . Mdm2 promotes the rapid degradation of p53. Nature 1997; 387: 296–299.
Wu Y, Chen L, Cao L, Sheng W, Yang BB . Overexpression of the C-terminal PG-M/versican domain impairs growth of tumor cells by intervening in the interaction between epidermal growth factor receptor and beta1-integrin. J Cell Sci 2004; 117: 2227–2237.
Rutnam ZJ, Du WW, Yang W, Yang X, Yang BB . The pseudogene TUSC2P promotes TUSC2 function by binding multiple microRNAs. Nat Commun 2014; 5: 2914.
Shan SW, Lee DY, Deng Z, Shatseva T, Jeyapalan Z, Du WW et al. MicroRNA MiR-17 retards tissue growth and represses fibronectin expression. Nat Cell Biol 2009; 11: 1031–1038.
Yang X, Du WW, Li H, Liu F, Khorshidi A, Rutnam ZJ et al. Both mature miR-17-5p and passenger strand miR-17-3p target TIMP3 and induce prostate tumor growth and invasion. Nucleic Acids Res 2013; 41: 9688–9704.
Tuszynska I, Magnus M, Jonak K, Dawson W, Bujnicki JM . NPDock: a web server for protein-nucleic acid docking. Nucleic Acids Res 2015; 43: W425–W430.
Pietal MJ, Szostak N, Rother KM, Bujnicki JM . RNAmap2D - calculation, visualization and analysis of contact and distance maps for RNA and protein-RNA complex structures. BMC Bioinformatics 2012; 13: 333.
Acknowledgements
This work was supported by CIHR Bridge Fund (PJT149083) to BBY, National Natural Science Foundation of China (NSFC; 81472454) to LF, and a Career Investigator Award (CI 7418) from the Heart and Stroke Foundation of Ontario to BBY. WWD is supported by a Postdoctoral Fellowship from the Breast Cancer Foundation of Ontario.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Edited by M Oren
Supplementary Information accompanies this paper on Cell Death and Differentiation website
Supplementary information
Rights and permissions
About this article
Cite this article
Du, W., Fang, L., Yang, W. et al. Induction of tumor apoptosis through a circular RNA enhancing Foxo3 activity. Cell Death Differ 24, 357–370 (2017). https://doi.org/10.1038/cdd.2016.133
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/cdd.2016.133
This article is cited by
-
Biogenesis, function and role of circular RNA in Gall Bladder Carcinoma-A narrative review
Surgical and Experimental Pathology (2025)
-
Regulatory roles of circular RNAs in Wnt and other oncogenic signaling pathways in breast cancer progression: a comprehensive review
European Journal of Medical Research (2025)
-
Circular RNA profiling identifies circ_0001522, circ_0001278, and circ_0001801 as predictors of unfavorable prognosis and drivers of triple-negative breast cancer hallmarks
Cell Death Discovery (2025)
-
Noncoding RNA (ncRNA)-mediated regulation of TLRs: critical regulator of inflammation in tumor microenvironment
Medical Oncology (2025)
-
The role and potential mechanisms of exosomes in the progression of hepatocellular carcinoma
Holistic Integrative Oncology (2025)