Abstract
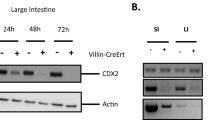
The intestinal epithelium undergoes a continual process of proliferation, differentiation and apoptosis. Previously, we have shown that the PI3K/Akt/mTOR pathway has a critical role in intestinal homeostasis. However, the downstream targets mediating the effects of mTOR in intestinal cells are not known. Here, we show that the ketone body β-hydroxybutyrate (βHB), an endogenous inhibitor of histone deacetylases (HDACs) induces intestinal cell differentiation as noted by the increased expression of differentiation markers (Mucin2 (MUC2), lysozyme, IAP, sucrase-isomaltase, KRT20, villin, Caudal-related homeobox transcription factor 2 (CDX2) and p21Waf1). Conversely, knockdown of the ketogenic mitochondrial enzyme hydroxymethylglutaryl CoA synthase 2 (HMGCS2) attenuated spontaneous differentiation in the human colon cancer cell line Caco-2. Overexpression of HMGCS2, which we found is localized specifically in the more differentiated portions of the intestinal mucosa, increased the expression of CDX2, thus further suggesting the contributory role of HMGCS2 in intestinal differentiation. In addition, mice fed a ketogenic diet demonstrated increased differentiation of intestinal cells as noted by an increase in the enterocyte, goblet and Paneth cell lineages. Moreover, we showed that either knockdown of mTOR or inhibition of mTORC1 with rapamycin increases the expression of HMGCS2 in intestinal cells in vitro and in vivo, suggesting a possible cross-talk between mTOR and HMGCS2/βHB signaling in intestinal cells. In contrast, treatment of intestinal cells with βHB or feeding mice with a ketogenic diet inhibits mTOR signaling in intestinal cells. Together, we provide evidence showing that HMGCS2/βHB contributes to intestinal cell differentiation. Our results suggest that mTOR acts cooperatively with HMGCS2/βHB to maintain intestinal homeostasis.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Yeung TM, Chia LA, Kosinski CM, Kuo CJ . Regulation of self-renewal and differentiation by the intestinal stem cell niche. Cell Mol Life Sci 2011; 68: 2513–2523.
De Mey JR, Freund JN . Understanding epithelial homeostasis in the intestine: an old battlefield of ideas, recent breakthroughs and remaining controversies. Tissue Barriers 2013; 1: e24965.
Gunther C, Neumann H, Neurath MF, Becker C . Apoptosis, necrosis and necroptosis: cell death regulation in the intestinal epithelium. Gut 2013; 62: 1062–1071.
Gersemann M, Wehkamp J, Stange EF . Innate immune dysfunction in inflammatory bowel disease. J Intern Med 2012; 271: 421–428.
Hammoud SS, Cairns BR, Jones DA . Epigenetic regulation of colon cancer and intestinal stem cells. Curr Opin Cell Biol 2013; 25: 177–183.
Clark JA, Doelle SM, Halpern MD, Saunders TA, Holubec H, Dvorak K et al. Intestinal barrier failure during experimental necrotizing enterocolitis: protective effect of EGF treatment. Am J Physiol Gastrointest Liver Physiol 2006; 291: G938–G949.
Shao J, Evers BM, Sheng H . Roles of phosphatidylinositol 3'-kinase and mammalian target of rapamycin/p70 ribosomal protein S6 kinase in K-Ras-mediated transformation of intestinal epithelial cells. Cancer Res 2004; 64: 229–235.
Wang Q, Zhou Y, Jackson LN, Johnson SM, Chow CW, Evers BM . Nuclear factor of activated T cells (NFAT) signaling regulates PTEN expression and intestinal cell differentiation. Mol Biol Cell 2011; 22: 412–420.
Zhou Y, Wang Q, Guo Z, Weiss HL, Evers BM . Nuclear factor of activated T-cell c3 inhibition of mammalian target of rapamycin signaling through induction of regulated in development and DNA damage response 1 in human intestinal cells. Mol Biol Cell 2012; 23: 2963–2972.
Wang Q, Zhou Y, Rychahou P, Liu C, Weiss HL, Evers BM . NFAT5 represses canonical Wnt signaling via inhibition of beta-catenin acetylation and participates in regulating intestinal cell differentiation. Cell Death Dis 2013; 4: e671.
Shyh-Chang N, Daley GQ, Cantley LC . Stem cell metabolism in tissue development and aging. Development 2013; 140: 2535–2547.
Stringari C, Edwards RA, Pate KT, Waterman ML, Donovan PJ, Gratton E . Metabolic trajectory of cellular differentiation in small intestine by phasor fluorescence lifetime microscopy of NADH. Sci Rep 2012; 2: 568.
Wang Q, Zhou Y, Wang X, Evers BM . p27 Kip1 nuclear localization and cyclin-dependent kinase inhibitory activity are regulated by glycogen synthase kinase-3 in human colon cancer cells. Cell Death Differ 2008; 15: 908–919.
Vanhoutvin SA, Troost FJ, Hamer HM, Lindsey PJ, Koek GH, Jonkers DM et al. Butyrate-induced transcriptional changes in human colonic mucosa. PLoS One 2009; 4: e6759.
Alexandre A . Suggested involvement of ketone bodies in the pathogenesis of the metabolic syndrome. Med Hypotheses 2013; 80: 578–581.
Newman JC, Verdin E . Ketone bodies as signaling metabolites. Trends Endocrinol Metab 2014; 25: 42–52.
Shimazu T, Hirschey MD, Newman J, He W, Shirakawa K, Le Moan N et al. Suppression of oxidative stress by beta-hydroxybutyrate, an endogenous histone deacetylase inhibitor. Science 2013; 339: 211–214.
Tou L, Liu Q, Shivdasani RA . Regulation of mammalian epithelial differentiation and intestine development by class I histone deacetylases. Mol Cell Biol 2004; 24: 3132–3139.
Kumar SP, Roy SJ, Tokumo K, Reddy BS . Effect of different levels of calorie restriction on azoxymethane-induced colon carcinogenesis in male F344 rats. Cancer Res 1990; 50: 5761–5766.
Lane AN, Fan TW, Higashi RM . Isotopomer-based metabolomic analysis by NMR and mass spectrometry. Biophys Tools Biologists 2008; 84: 541–588.
Wang Q, Wang X, Hernandez A, Kim S, Evers BM . Inhibition of the phosphatidylinositol 3-kinase pathway contributes to HT29 and Caco-2 intestinal cell differentiation. Gastroenterology 2001; 120: 1381–1392.
Mak AB, Nixon AM, Kittanakom S, Stewart JM, Chen GI, Curak J et al. Regulation of CD133 by HDAC6 promotes beta-catenin signaling to suppress cancer cell differentiation. Cell Rep 2012; 2: 951–963.
Evers BM . Intestinal cell differentiation: cellular mechanisms and the search for the perfect model focus on "involvement of p21(WAF1/Cip1) and p27(Kip1) in intestinal epithelial cell differentiation". Am J Physiol 1999; 276 (6 Pt 1): C1243–C1244.
Saad RS, Ghorab Z, Khalifa MA, Xu M . CDX2 as a marker for intestinal differentiation: its utility and limitations. World J Gastrointest Surg 2011; 3: 159–166.
Hegardt FG . Mitochondrial 3-hydroxy-3-methylglutaryl-CoA synthase: a control enzyme in ketogenesis. Biochem J 1999; 338Pt 3569–582.
Schwartz B, Avivi-Green C, Polak-Charcon S . Sodium butyrate induces retinoblastoma protein dephosphorylation, p16 expression and growth arrest of colon cancer cells. Mol Cell Biochem 1998; 188: 21–30.
Burger-van Paassen N, Vincent A, Puiman PJ, van der Sluis M, Bouma J, Boehm G et al. The regulation of intestinal mucin MUC2 expression by short-chain fatty acids: implications for epithelial protection. Biochem J 2009; 420: 211–219.
Heuberger J, Kosel F, Qi J, Grossmann KS, Rajewsky K, Birchmeier W . Shp2/MAPK signaling controls goblet/paneth cell fate decisions in the intestine. Proc Natl Acad Sci USA 2014; 111: 3472–3477.
Helenius TO, Misiorek JO, Nystrom JH, Fortelius LE, Habtezion A, Liao J et al. Keratin 8 absence down-regulates colonocyte HMGCS2 and modulates colonic ketogenesis and energy metabolism. Mol Biol Cell 2015; 26: 2298–2310.
Youm YH, Nguyen KY, Grant RW, Goldberg EL, Bodogai M, Kim D et al. The ketone metabolite beta-hydroxybutyrate blocks NLRP3 inflammasome-mediated inflammatory disease. Nat Med 2015; 21: 263–269.
Tate RL, Mehlman MA, Tobin RB . Metabolic fate of 1,3-butanediol in the rat: conversion to -hydroxybutyrate. J Nutr 1971; 101: 1719–1726.
Sengupta S, Peterson TR, Laplante M, Oh S, Sabatini DM . mTORC1 controls fasting-induced ketogenesis and its modulation by ageing. Nature 2010; 468: 1100–1104.
Zhou Y, Wang Q, Weiss HL, Evers BM . Nuclear factor of activated T-cells 5 increases intestinal goblet cell differentiation through an mTOR/Notch signaling pathway. Mol Biol Cell 2014; 25: 2882–2890.
Zhou Y, Rychahou P, Wang Q, Weiss HL, Evers BM . TSC2/mTORC1 signaling controls Paneth and goblet cell differentiation in the intestinal epithelium. Cell Death Dis 2015; 6: e1631.
Newman JC, Verdin E . beta-hydroxybutyrate: much more than a metabolite. Diabetes Res Clin Pract 2014; 106: 173–181.
Zavitz KH, Zipursky SL . Controlling cell proliferation in differentiating tissues: genetic analysis of negative regulators of G1–->S-phase progression. Curr Opin Cell Biol 1997; 9: 773–781.
Kaiko GE, Ryu SH, Koues OI, Collins PL, Solnica-Krezel L, Pearce EJ et al. The colonic crypt protects stem cells from microbiota-derived metabolites. Cell 2016; 165: 1708–1720.
Shen Y, Wei W, Zhou DX . Histone acetylation enzymes coordinate metabolism and gene expression. Trends Plant Sci 2015; 20: 614–621.
Roostaee A, Benoit YD, Boudjadi S, Beaulieu JF . Epigenetics in intestinal epithelial cell renewal. J Cell Physiol 2016; 231: 2361–2367.
Stringer EJ, Duluc I, Saandi T, Davidson I, Bialecka M, Sato T et al. Cdx2 determines the fate of postnatal intestinal endoderm. Development 2012; 139: 465–474.
Hryniuk A, Grainger S, Savory JG, Lohnes D . Cdx function is required for maintenance of intestinal identity in the adult. Dev Biol 2012; 363: 426–437.
Domon-Dell C, Wang Q, Kim S, Kedinger M, Evers BM, Freund JN . Stimulation of the intestinal Cdx2 homeobox gene by butyrate in colon cancer cells. Gut 2002; 50: 525–529.
Bai YQ, Miyake S, Iwai T, Yuasa Y . CDX2, a homeobox transcription factor, upregulates transcription of the p21/WAF1/CIP1 gene. Oncogene 2003; 22: 7942–7949.
Gulhati P, Cai Q, Li J, Liu J, Rychahou PG, Qiu S et al. Targeted inhibition of mammalian target of rapamycin signaling inhibits tumorigenesis of colorectal cancer. Clin Cancer Res 2009; 15: 7207–7216.
Wang Q, Zhou Y, Wang X, Chung DH, Evers BM . Regulation of PTEN expression in intestinal epithelial cells by c-Jun NH2-terminal kinase activation and nuclear factor-kappaB inhibition. Cancer Res 2007; 67: 7773–7781.
Camarero N, Mascaro C, Mayordomo C, Vilardell F, Haro D, Marrero PF . Ketogenic HMGCS2 Is a c-Myc target gene expressed in differentiated cells of human colonic epithelium and down-regulated in colon cancer. Mol Cancer Res 2006; 4: 645–653.
Fu Z, Kim J, Vidrich A, Sturgill TW, Cohn SM . Intestinal cell kinase, a MAP kinase-related kinase, regulates proliferation and G1 cell cycle progression of intestinal epithelial cells. Am J Physiol Gastrointest Liver Physiol 2009; 297: G632–G640.
McDaniel SS, Rensing NR, Thio LL, Yamada KA, Wong M . The ketogenic diet inhibits the mammalian target of rapamycin (mTOR) pathway. Epilepsia 2011; 52: e7–11.
Zhang P, Guo Z, Wu Y, Hu R, Du J, He X et al. Histone deacetylase inhibitors inhibit the proliferation of gall bladder carcinoma cells by suppressing AKT/mTOR signaling. PLoS One 2015; 10: e0136193.
Gonneaud A, Turgeon N, Boudreau F, Perreault N, Rivard N, Asselin C . Distinct roles for intestinal epithelial cell-specific Hdac1 and Hdac2 in the regulation of murine intestinal homeostasis. J Cell Physiol 2016; 231: 436–448.
Helmrath MA, Fong JJ, Dekaney CM, Henning SJ . Rapid expansion of intestinal secretory lineages following a massive small bowel resection in mice. Am J Physiol Gastrointest Liver Physiol 2007; 292: G215–G222.
Sadanandam A, Lyssiotis CA, Homicsko K, Collisson EA, Gibb WJ, Wullschleger S et al. A colorectal cancer classification system that associates cellular phenotype and responses to therapy. Nat Med 2013; 19: 619–625.
Acknowledgements
We thank HN Russell-Simmons for manuscript preparation; D Napier for tissue sectioning and staining; EY Lee for consultation and assessment of histological sections and IHC; the Biospecimen and Tissue Procurement, Redox Metabolism, and Biostatistics and Bioinformatics Shared Resource Facilities of the University of Kentucky Markey Cancer Center (supported by National Cancer Institute grant P30 CA177558). This work was further supported by National Institutes of Health grant R01 DK48498. MS and NMR analyses were carried out in the CESB facilities with support, in part, from U24 DK097215 (RM Higashi, Program Director)
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Edited by E Gottlieb
Supplementary Information accompanies this paper on Cell Death and Differentiation website
Rights and permissions
About this article
Cite this article
Wang, Q., Zhou, Y., Rychahou, P. et al. Ketogenesis contributes to intestinal cell differentiation. Cell Death Differ 24, 458–468 (2017). https://doi.org/10.1038/cdd.2016.142
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/cdd.2016.142
This article is cited by
-
miR-181a-5p mediates the effects of BMP4 on intestinal cell proliferation and differentiation
Cell Death & Disease (2025)
-
Sympathetic-Mediated Intestinal Cell Death Contributes to Gut Barrier Impairment After Stroke
Translational Stroke Research (2025)
-
Death receptor 5 is required for intestinal stem cell activity during intestinal epithelial renewal at homoeostasis
Cell Death & Disease (2024)
-
Age-, sex- and proximal–distal-resolved multi-omics identifies regulators of intestinal aging in non-human primates
Nature Aging (2024)
-
Targeting lysine-specific demethylase 1 (KDM1A/LSD1) impairs colorectal cancer tumorigenesis by affecting cancer cells stemness, motility, and differentiation
Cell Death Discovery (2023)