Abstract
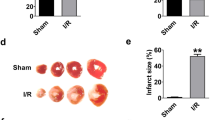
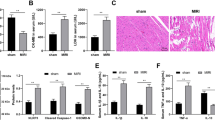
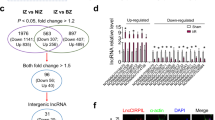
Emerging evidences suggest that necrosis is programmed and is one of the main forms of cell death in the pathological process in cardiac diseases. Long noncoding RNAs (lncRNAs) are emerging as new players in gene regulation. However, it is not yet clear whether lncRNAs can regulate necrosis in cardiomyocytes. Here, we report that a long noncoding RNA, named necrosis-related factor (NRF), regulates cardiomyocytes necrosis by targeting miR-873 and RIPK1 (receptor-interacting serine/threonine-protein kinase 1)/RIPK3 (receptor-interacting serine/threonine-protein kinase 3). Our results show that RIPK1 and RIPK3 participate in H2O2-induced cardiomyocytes necrosis. miR-873 suppresses the translation of RIPK1/RIPK3 and inhibits RIPK1/RIPK3-mediated necrotic cell death in cardiomyocytes. miR-873 reduces myocardial infarct size upon ischemia/reperfusion (I/R) injury in the animal model. In exploring the molecular mechanism by which miR-873 expression is regulated, we identify NRF as an endogenous sponge RNA and repress miR-873 expression. NRF directly binds to miR-873 and regulates RIPK1/RIPK3 expression and necrosis. Knockdown of NRF antagonizes necrosis in cardiomyocytes and reduces necrosis and myocardial infarction upon I/R injury. Further, we identify that p53 transcriptionally activates NRF expression. P53 regulates cardiomyocytes necrosis and myocardial I/R injury through NRF and miR-873.Our results identify a novel mechanism involving NRF and miR-873 in regulating programmed necrosis in the heart and suggest a potential therapeutic avenue for cardiovascular diseases.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
Abbreviations
- lncRNA:
-
long noncoding RNA
- miRNAs:
-
microRNAs
- H2O2:
-
hydrogen peroxide
- I/R:
-
ischemia/reperfusion
- PI:
-
propidium iodide
- RIPK1:
-
receptor-interacting serine/threonine-protein kinase 1
- RIPK3:
-
receptor-interacting serine/threonine-protein kinase 3
- qRT-PCR:
-
quantitative reverse transcription polymerase chain reaction
- TP:
-
target protector
References
Cho YS, Park SY, Shin HS, Chan FK . Physiological consequences of programmed necrosis, an alternative form of cell demise. Mol Cells 2010; 29: 327–332.
Lamkanfi M, Festjens N, Declercq W, Vanden Berghe T, Vandenabeele P . Caspases in cell survival, proliferation and differentiation. Cell Death Differ 2007; 14: 44–55.
Nakagawa T, Shimizu S, Watanabe T, Yamaguchi O, Otsu K, Yamagata H et al. Cyclophilin d-dependent mitochondrial permeability transition regulates some necrotic but not apoptotic cell death. Nature 2005; 434: 652–658.
Baines CP, Kaiser RA, Purcell NH, Blair NS, Osinska H, Hambleton MA et al. Loss of cyclophilin d reveals a critical role for mitochondrial permeability transition in cell death. Nature 2005; 434: 658–662.
Oerlemans MI, Liu J, Arslan F, den Ouden K, van Middelaar BJ, Doevendans PA et al. Inhibition of rip1-dependent necrosis prevents adverse cardiac remodeling after myocardial ischemia-reperfusion in vivo. Basic Res Cardiol 2012; 107: 270.
Kingma JG Jr, Yellon DM . Inability of dimethylthiourea to limit tissue necrosis during acute myocardial infarction in rabbits. Free Radic Biol Med 1992; 12: 263–270.
Lissoni P, Pelizzoni F, Mauri O, Perego M, Pittalis S, Barni S . Enhanced secretion of tumour necrosis factor in patients with myocardial infarction. Eur J Med 1992; 1: 277–280.
Bryant D, Becker L, Richardson J, Shelton J, Franco F, Peshock R et al. Cardiac failure in transgenic mice with myocardial expression of tumor necrosis factor-alpha. Circulation 1998; 97: 1375–1381.
Guerra S, Leri A, Wang X, Finato N, Di Loreto C, Beltrami CA et al. Myocyte death in the failing human heart is gender dependent. Circ Res 1999; 85: 856–866.
Cho YS, Challa S, Moquin D, Genga R, Ray TD, Guildford M et al. Phosphorylation-driven assembly of the rip1-rip3 complex regulates programmed necrosis and virus-induced inflammation. Cell 2009; 137: 1112–1123.
Zhang DW, Shao J, Lin J, Zhang N, Lu BJ, Lin SC et al. Rip3, an energy metabolism regulator that switches tnf-induced cell death from apoptosis to necrosis. Science 2009; 325: 332–336.
He S, Wang L, Miao L, Wang T, Du F, Zhao L et al. Receptor interacting protein kinase-3 determines cellular necrotic response to tnf-alpha. Cell 2009; 137: 1100–1111.
Sun L, Wang H, Wang Z, He S, Chen S, Liao D et al. Mixed lineage kinase domain-like protein mediates necrosis signaling downstream of rip3 kinase. Cell 2012; 148: 213–227.
Luedde M, Lutz M, Carter N, Sosna J, Jacoby C, Vucur M et al. Rip3, a kinase promoting necroptotic cell death, mediates adverse remodelling after myocardial infarction. Cardiovasc Res 2014; 103: 206–216.
Lee Y, Ahn C, Han J, Choi H, Kim J, Yim J et al. The nuclear rnase iii drosha initiates microrna processing. Nature 2003; 425: 415–419.
Ohtani K, Dimmeler S . Control of cardiovascular differentiation by micrornas. Basic Res Cardiol 2011; 106: 5–11.
Qian L, Van Laake LW, Huang Y, Liu S, Wendland MF, Srivastava D . Mir-24 inhibits apoptosis and represses bim in mouse cardiomyocytes. J Exp Med 2011; 208: 549–560.
Wang X, Zhang X, Ren XP, Chen J, Liu H, Yang J et al. Microrna-494 targeting both proapoptotic and antiapoptotic proteins protects against ischemia/reperfusion-induced cardiac injury. Circulation 2010; 122: 1308–1318.
Sayed D, He M, Hong C, Gao S, Rane S, Yang Z et al. Microrna-21 is a downstream effector of akt that mediates its antiapoptotic effects via suppression of fas ligand. J Biol Chem 2010; 285: 20281–20290.
Li P . Micrornas in cardiac apoptosis. J Cardiovasc Transl Res 2010; 3: 219–224.
Basson M . Micrornas loom large in the heart. Nat Med 2007; 13: 541.
Zhao Y, Ransom JF, Li A, Vedantham V, von Drehle M, Muth AN et al. Dysregulation of cardiogenesis, cardiac conduction, and cell cycle in mice lacking mirna-1-2. Cell 2007; 129: 303–317.
Yang B, Lin H, Xiao J, Lu Y, Luo X, Li B et al. The muscle-specific microrna mir-1 regulates cardiac arrhythmogenic potential by targeting gja1 and kcnj2. Nat Med 2007; 13: 486–491.
Thum T, Galuppo P, Wolf C, Fiedler J, Kneitz S, van Laake LW et al. Micrornas in the human heart: a clue to fetal gene reprogramming in heart failure. Circulation 2007; 116: 258–267.
Chien KR . Molecular medicine: micrornas and the tell-tale heart. Nature 2007; 447: 389–390.
Gupta RA, Shah N, Wang KC, Kim J, Horlings HM, Wong DJ et al. Long non-coding rna hotair reprograms chromatin state to promote cancer metastasis. Nature 2010; 464: 1071–1076.
Gong C, Maquat LE . Lncrnas transactivate stau1-mediated mrna decay by duplexing with 3' utrs via alu elements. Nature 2011; 470: 284–288.
Clemson CM, Hutchinson JN, Sara SA, Ensminger AW, Fox AH, Chess A et al. An architectural role for a nuclear noncoding rna: Neat1 rna is essential for the structure of paraspeckles. Mol Cell 2009; 33: 717–726.
Khaitan D, Dinger ME, Mazar J, Crawford J, Smith MA, Mattick JS et al. The melanoma-upregulated long noncoding rna spry4-it1 modulates apoptosis and invasion. Cancer Res 2011; 71: 3852–3862.
Ginger MR, Shore AN, Contreras A, Rijnkels M, Miller J, Gonzalez-Rimbau MF et al. A noncoding rna is a potential marker of cell fate during mammary gland development. Proc Natl Acad Sci USA 2006; 103: 5781–5786.
Archer K, Broskova Z, Bayoumi AS, Teoh JP, Davila A, Tang Y et al. Long non-coding rnas as master regulators in cardiovascular diseases. Int J Mol Sci 2015; 16: 23651–23667.
Ounzain S, Burdet F, Ibberson M, Pedrazzini T . Discovery and functional characterization of cardiovascular long noncoding rnas. J Mol Cell Cardiol 2015; 89: 17–26.
Gao W, Wang ZM, Zhu M, Lian XQ, Zhao H, Zhao D et al. Altered long noncoding rna expression profiles in the myocardium of rats with ischemic heart failure. J Cardiovasc Med (Hagerstown, MD) 2015; 16: 473–479.
Pinet F, Bauters C . Potential of non-coding rna as biomarkers in heart failure. Med Sci (Paris) 2015; 31: 770–776.
Gardner AM, Xu FH, Fady C, Jacoby FJ, Duffey DC, Tu Y et al. Apoptotic vs. nonapoptotic cytotoxicity induced by hydrogen peroxide. Free Radic Biol Med 1997; 22: 73–83.
Takeda M, Shirato I, Kobayashi M, Endou H . Hydrogen peroxide induces necrosis, apoptosis, oncosis and apoptotic oncosis of mouse terminal proximal straight tubule cells. Nephron 1999; 81: 234–238.
Troyano A, Sancho P, Fernandez C, de Blas E, Bernardi P, Aller P . The selection between apoptosis and necrosis is differentially regulated in hydrogen peroxide-treated and glutathione-depleted human promonocytic cells. Cell Death Differ 2003; 10: 889–898.
Downey JM . Free radicals and their involvement during long-term myocardial ischemia and reperfusion. Annu Rev Physiol 1990; 52: 487–504.
Cesana M, Cacchiarelli D, Legnini I, Santini T, Sthandier O, Chinappi M et al. A long noncoding rna controls muscle differentiation by functioning as a competing endogenous rna. Cell 2011; 147: 358–369.
Wang J, Liu X, Wu H, Ni P, Gu Z, Qiao Y et al. Creb up-regulates long non-coding rna, hulc expression through interaction with microrna-372 in liver cancer. Nucleic Acids Res 2010; 38: 5366–5383.
Cazalla D, Yario T, Steitz JA . Down-regulation of a host microrna by a herpesvirus saimiri noncoding rna. Science 2010; 328: 1563–1566.
Liu X, He F, Pang R, Zhao D, Qiu W, Shan K et al. Interleukin-17 (il-17)-induced microrna 873 (mir-873) contributes to the pathogenesis of experimental autoimmune encephalomyelitis by targeting a20 ubiquitin-editing enzyme. J Biol Chem 2014; 289: 28971–28986.
Rahman S, Quann K, Pandya D, Singh S, Khan ZK, Jain P . Htlv-1 tax mediated downregulation of mirnas associated with chromatin remodeling factors in t cells with stably integrated viral promoter. PloS One 2012; 7: e34490.
Skalsky RL, Cullen BR . Reduced expression of brain-enriched micrornas in glioblastomas permits targeted regulation of a cell death gene. PloS One 2011; 6: e24248.
Morgan CP, Bale TL . Early prenatal stress epigenetically programs dysmasculinization in second-generation offspring via the paternal lineage. J Neurosci 2011; 31: 11748–11755.
Holler N, Zaru R, Micheau O, Thome M, Attinger A, Valitutti S et al. Fas triggers an alternative, caspase-8-independent cell death pathway using the kinase rip as effector molecule. Nat Immunol 2000; 1: 489–495.
Vercammen D, Beyaert R, Denecker G, Goossens V, Van Loo G, Declercq W et al. Inhibition of caspases increases the sensitivity of l929 cells to necrosis mediated by tumor necrosis factor. J Exp Med 1998; 187: 1477–1485.
Remijsen Q, Goossens V, Grootjans S, Van den Haute C, Vanlangenakker N, Dondelinger Y et al. Depletion of ripk3 or mlkl blocks tnf-driven necroptosis and switches towards a delayed ripk1 kinase-dependent apoptosis. Cell Death Dis 2014; 5: e1004.
Eum HA, Vallabhaneni R, Wang Y, Loughran PA, Stolz DB, Billiar TR . Characterization of disc formation and tnfr1 translocation to mitochondria in tnf-alpha-treated hepatocytes. Am J Pathol 2011; 179: 1221–1229.
Cusson N, Oikemus S, Kilpatrick ED, Cunningham L, Kelliher M . The death domain kinase rip protects thymocytes from tumor necrosis factor receptor type 2-induced cell death. J Exp Med 2002; 196: 15–26.
Bellail AC, Tse MC, Song JH, Phuphanich S, Olson JJ, Sun SY et al. Dr5-mediated disc controls caspase-8 cleavage and initiation of apoptosis in human glioblastomas. J Cell Mol Med 2010; 14: 1303–1317.
Kanduri C . Kcnq1ot1: a chromatin regulatory rna. Semin Cell Dev Biol 2011; 22: 343–350.
Kumar MS, Armenteros-Monterroso E, East P, Chakravorty P, Matthews N, Winslow MM et al. Hmga2 functions as a competing endogenous rna to promote lung cancer progression. Nature 2014; 505: 212–217.
Krutzfeldt J, Rajewsky N, Braich R, Rajeev KG, Tuschl T, Manoharan M et al. Silencing of micrornas in vivo with 'antagomirs'. Nature 2005; 438: 685–689.
Jimenez-Mateos EM, Engel T, Merino-Serrais P, McKiernan RC, Tanaka K, Mouri G et al. Silencing microrna-134 produces neuroprotective and prolonged seizure-suppressive effects. Nat Med 2012; 18: 1087–1094.
Horwich MD, Zamore PD . Design and delivery of antisense oligonucleotides to block microrna function in cultured drosophila and human cells. Nat Protoc 2008; 3: 1537–1549.
Wei C-L, Wu Q, Vega VB, Chiu KP, Ng P, Zhang T et al. A global map of p53 transcription-factor binding sites in the human genome. Cell 2006; 124: 207–219.
Vaseva AV, Marchenko ND, Ji K, Tsirka SE, Holzmann S, Moll UM . P53 opens the mitochondrial permeability transition pore to trigger necrosis. Cell 2012; 149: 1536–1548.
Furukawa A, Tada-Oikawa S, Kawanishi S, Oikawa S . H2o2 accelerates cellular senescence by accumulation of acetylated p53 via decrease in the function of sirt1 by nad+ depletion. Cell Physiol Biochem 2007; 20: 45–54.
Wang Y-H, Tsay Y-G, Tan BC-M, Lo W-Y, Lee S-C . Identification and characterization of a novel p300-mediated p53 acetylation site, lysine 305. J Biol Chem 2003; 278: 25568–25576.
Xie S, Wang Q, Wu H, Cogswell J, Lu L, Jhanwar-Uniyal M et al. Reactive oxygen species-induced phosphorylation of p53 on serine 20 is mediated in part by polo-like kinase-3. J Biol Chem 2001; 276: 36194–36199.
Uberti D, Yavin E, Gil S, Ayasola K-R, Goldfinger N, Rotter V . Hydrogen peroxide induces nuclear translocation of p53 and apoptosis in cells of oligodendroglia origin. Mol Brain Res 1999; 65: 167–175.
Long X, Goldenthal MJ, Marín-García J . Oxidative stress enhances phosphorylation of p53 in neonatal rat cardiomyocytes. Mol Cell Biochem 2007; 303: 167–174.
Tan WQ, Wang K, Lv DY, Li PF . Foxo3a inhibits cardiomyocyte hypertrophy through transactivating catalase. J Biol Chem 2008; 283: 29730–29739.
Lin Z, Murtaza I, Wang K, Jiao J, Gao J, Li PF . Mir-23a functions downstream of nfatc3 to regulate cardiac hypertrophy. Proc Natl Acad Sci USA 2009; 106: 12103–12108.
Chen C, Ridzon DA, Broomer AJ, Zhou Z, Lee DH, Nguyen JT et al. Real-time quantification of micrornas by stem-loop rt-pcr. Nucleic Acids Res 2005; 33: e179.
Li PF, Dietz R, von Harsdorf R . P53 regulates mitochondrial membrane potential through reactive oxygen species and induces cytochrome c-independent apoptosis blocked by bcl-2. EMBO J 1999; 18: 6027–6036.
Wang JX, Jiao JQ, Li Q, Long B, Wang K, Liu JP et al. Mir-499 regulates mitochondrial dynamics by targeting calcineurin and dynamin-related protein-1. Nat Med 2011; 17: 71–78.
Acknowledgements
This work was supported by National Natural Science Foundation of China (81522005, 81270160, 81470522) and Beijing Natural Science Foundation (7142103) and Natural Science Foundation of Shandong Province (BS2014SW028).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Edited by V Stambolic
Supplementary Information accompanies this paper on Cell Death and Differentiation website
Supplementary information
Rights and permissions
About this article
Cite this article
Wang, K., Liu, F., Liu, CY. et al. The long noncoding RNA NRF regulates programmed necrosis and myocardial injury during ischemia and reperfusion by targeting miR-873. Cell Death Differ 23, 1394–1405 (2016). https://doi.org/10.1038/cdd.2016.28
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/cdd.2016.28
This article is cited by
-
Cuproptosis: p53-regulated metabolic cell death?
Cell Death & Differentiation (2023)
-
The Role of P53 in Myocardial Ischemia-Reperfusion Injury
Cardiovascular Drugs and Therapy (2023)
-
The Effects of MicroRNAs in the Development of Heart Failure
Current Cardiology Reports (2023)
-
LncRNA LINC00461 exacerbates myocardial ischemia–reperfusion injury via microRNA-185-3p/Myd88
Molecular Medicine (2022)
-
LncRNA XR_596701 protects H9c2 cells against intermittent hypoxia-induced injury through regulation of the miR-344b-5p/FAIM3 axis
Cell Death Discovery (2022)