Abstract
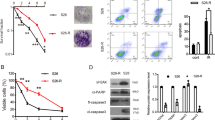
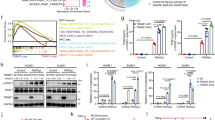
Radioresistance is a major obstacle in successful clinical cancer radiotherapy, and the underlying mechanisms are not clear. Here we show that IKKα-mediated miR-196a biogenesis via interaction with Drosha regulates the sensitivity of nasopharyngeal carcinoma (NPC) cells to radiotherapy. Phosphorylation of IKKα at T23 site (p-IKKαT23) promotes the binding of IKKα to Drosha that accelerates the processing of miR-196a primary transcripts, leading to increased expressions of both precursor and mature miR-196a. Dephosphorylation of p-IKKαT23 downregulates miR-196a expression and promotes the resistance of NPC cells to radiation treatment. The miR-196a mimic suppresses while its inhibitor promotes the resistance of NPC to radiation treatment. Importantly, the expression of p-IKKαT23 is positively related to the expression of miR-196a in human NPC tissues, and expression of p-IKKαT23 and miR-196a is inversely correlated with NPC clinical radioresistance. Thus, our studies establish a novel mechanistic link between the inactivation of IKKαT23–Drosha–miR-196a pathway and NPC radioresistance, and de-inactivation of IKKαT23–Drosha–miR-196a pathway would be an efficient way to restore the sensitivity of radioresistant NPC to radiotherapy.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
Abbreviations
- ATM:
-
ataxia telangiectasia-mutated protein kinase
- BRCA1:
-
Breast cancer susceptibility gene 1
- CHK2:
-
Checkpoint kinase 2
- DDX5:
-
DEAD (Asp-Glu-Ala-Asp) box helicase 5
- DDX17:
-
DEAD-box helicase 17
- DHX9:
-
DEAH (Asp-Glu-Ala-His) Box Helicase 9
- DGCR8:
-
DiGeorge syndrome critical region gene 8
- EMSA:
-
electrophoretic mobility shift assay
- hnRNP A1:
-
heterogeneous nuclear ribonucleoprotein A1
- IKK:
-
IκB kinase
- IR:
-
ionizing radiation
- NF-κB:
-
nuclear factor kappa-light-chain-enhancer of activated B cells
- NPC:
-
nasopharyngeal carcinoma
- PI3K:
-
phosphoinositide 3-kinase
- RIP:
-
RNA immunoprecipitation
- Smad:
-
Sma and Mad
References
Yu MC, Yuan JM . Epidemiology of nasopharyngeal carcinoma. Semin Cancer Biol 2002; 12: 421–429.
Lee AW, Poon YF, Foo W, Law SC, Cheung FK, Chan DK et al. Retrospective analysis of 5037 patients with nasopharyngeal carcinoma treated during 1976-1985: overall survival and patterns of failure. Int J Radiat Oncol Biol Phys 1992; 23: 261–270.
Hui EP, Leung SF, Au JS, Zee B, Tung S, Chua D et al. Lung metastasis alone in nasopharyngeal carcinoma: a relatively favorable prognostic group. A study by the Hong Kong Nasopharyngeal Carcinoma Study Group. Cancer 2004; 101: 300–306.
Wang WJ, Wu SP, Liu JB, Shi YS, Huang X, Zhang QB et al. MYC regulation of CHK1 and CHK2 promotes radioresistance in a stem cell-like population of nasopharyngeal carcinoma cells. Cancer Res 2013; 73: 1219–1231.
Rothwarf DM, Karin M . The NF-kappa B activation pathway: a paradigm in information transfer from membrane to nucleus. Sci STKE 1999; 1999: RE1.
Karin M, Ben-Neriah Y . Phosphorylation meets ubiquitination: the control of NF-[kappa]B activity. Annu Rev Immunol 2000; 18: 621–663.
Greten FR, Karin M . NF-kB: linking inflammation and immunity to cancer development and progression. Nat Rev Immunol 2005; 5: 749–759.
Luo JL, Kamata H, Karin M . IKK/NF-kappaB signaling: balancing life and death—a new approach to cancer therapy. J Clin Invest 2005; 115: 2625–2632.
Ghosh S, Karin M . Missing pieces in the NF-kB puzzle. Cell 2002; 109 (Suppl):S81–S96.
Bonizzi G, Karin M . The two NF-kB activation pathways and their role in innate and adaptive immunity. Trends Immunol 2004; 25: 280–288.
Senftleben U, Cao Y, Xiao G, Greten FR, Krahn G, Bonizzi G et al. Activation by IKKa of a second, evolutionary conserved, NF-kB signaling pathway. Science 2001; 293: 1495–1499.
Bonizzi G, Bebien M, Otero DC, Johnson-Vroom KE, Cao Y, Vu D et al. Activation of IKKa target genes depends on recognition of specific kB binding sites by RelB:p52 dimers. EMBO J 2004; 23: 4202–4210.
Luo JL, Tan W, Ricono JM, Korchynskyi O, Zhang M, Gonias SL et al. Nuclear cytokine-activated IKKalpha controls prostate cancer metastasis by repressing Maspin. Nature 2007; 446: 690–694.
Ammirante M, Luo JL, Grivennikov S, Nedospasov S, Karin M . B-cell-derived lymphotoxin promotes castration-resistant prostate cancer. Nature 2010; 464: 302–305.
Chen K, Rajewsky N . The evolution of gene regulation by transcription factors and microRNAs. Nat Rev Genet 2007; 8: 93–103.
Davis BN, Hilyard AC, Lagna G, Hata A . SMAD proteins control DROSHA-mediated microRNA maturation. Nature 2008; 454: 56–61.
Kawai S, Amano A . BRCA1 regulates microRNA biogenesis via the DROSHA microprocessor complex. J Cell Biol 2012; 197: 201–208.
Suzuki HI, Yamagata K, Sugimoto K, Iwamoto T, Kato S, Miyazono K . Modulation of microRNA processing by p53. Nature 2009; 460: 529–533.
Yan M, Zhang Y, He B, Xiang J, Wang ZF, Zheng FM et al. IKKalpha restoration via EZH2 suppression induces nasopharyngeal carcinoma differentiation. Nat Commun 2014; 5: 3661.
Deng L, Li Y, Ai P, Xie Y, Zhu H, Chen N . Increase in IkappaB kinase alpha expression suppresses the tumor progression and improves the prognosis for nasopharyngeal carcinoma. Mol Carcinog 2013; 54: 156–165.
Tang X, Zhang Y, Tucker L, Ramratnam B . Phosphorylation of the RNase III enzyme Drosha at Serine300 or Serine302 is required for its nuclear localization. Nucleic Acids Res 2010; 38: 6610–6619.
Besse A, Sana J, Fadrus P, Slaby O . MicroRNAs involved in chemo- and radioresistance of high-grade gliomas. Tumour Biol 2013; 34: 1969–1978.
Czochor JR, Glazer PM . microRNAs in cancer cell response to ionizing radiation. Antioxid Redox Signal 2014; 21: 293–312.
Carthew RW, Sontheimer EJ . Origins and mechanisms of miRNAs and siRNAs. Cell 2009; 136: 642–655.
Kim VN, Han J, Siomi MC . Biogenesis of small RNAs in animals. Nat Rev Mol Cell Biol 2009; 10: 126–139.
Ling H, Fabbri M, Calin GA . MicroRNAs and other non-coding RNAs as targets for anticancer drug development. Nat Rev Drug Discov 2013; 12: 847–865.
Gregory RI, Yan KP, Amuthan G, Chendrimada T, Doratotaj B, Cooch N et al. The microprocessor complex mediates the genesis of microRNAs. Nature 2004; 432: 235–240.
Morlando M, Ballarino M, Gromak N, Pagano F, Bozzoni I, Proudfoot NJ . Primary microRNA transcripts are processed co-transcriptionally. Nat Struct Mol Biol 2008; 15: 902–909.
Han J, Lee Y, Yeom KH, Kim YK, Jin H, Kim VN . The Drosha-DGCR8 complex in primary microRNA processing. Genes Dev 2004; 18: 3016–3027.
Michlewski G, Guil S, Semple CA, Caceres JF . Posttranscriptional regulation of miRNAs harboring conserved terminal loops. Mol Cell 2008; 32: 383–393.
Viswanathan SR, Daley GQ, Gregory RI . Selective blockade of microRNA processing by Lin28. Science 2008; 320: 97–100.
Davis BN, Hilyard AC, Nguyen PH, Lagna G, Hata A . Smad proteins bind a conserved RNA sequence to promote microRNA maturation by Drosha. Mol Cell 2010; 39: 373–384.
Anest V, Hanson JL, Cogswell PC, Steinbrecher KA, Strahl BD, Baldwin AS . A nucleosomal function for IkB kinase-a in NF-kB-dependent gene expression. Nature 2003; 423: 659–663.
Yamamoto Y, Verma UN, Prajapati S, Kwak YT, Gaynor RB . Histone H3 phosphorylation by IKKa is critical for cytokine-induced gene expression. Nature 2003; 423: 655–659.
Park KJ, Krishnan V, O'Malley BW, Yamamoto Y, Gaynor RB . Formation of an IKKalpha-dependent transcription complex is required for estrogen receptor-mediated gene activation. Mol Cell 2005; 18: 71–82.
Chen C, Zhang Y, Zhang L, Weakley SM, Yao Q . MicroRNA-196: critical roles and clinical applications in development and cancer. J Cell Mol Med 2011; 15: 14–23.
Schimanski CC, Frerichs K, Rahman F, Berger M, Lang H, Galle PR et al. High miR-196a levels promote the oncogenic phenotype of colorectal cancer cells. World J Gastroenterol 2009; 15: 2089–2096.
Wang YX, Zhang XY, Zhang BF, Yang CQ, Chen XM, Gao HJ . Initial study of microRNA expression profiles of colonic cancer without lymph node metastasis. J Dig Dis 2010; 11: 50–54.
Braig S, Mueller DW, Rothhammer T, Bosserhoff AK . MicroRNA miR-196a is a central regulator of HOX-B7 and BMP4 expression in malignant melanoma. Cell Mol Life Sci 2010; 67: 3535–3548.
Li Y, Zhang M, Chen H, Dong Z, Ganapathy V, Thangaraju M et al. Ratio of miR-196 s to HOXC8 messenger RNA correlates with breast cancer cell migration and metastasis. Cancer Res 2010; 70: 7894–7904.
Kim YJ, Bae SW, Yu SS, Bae YC, Jung JS . miR-196a regulates proliferation and osteogenic differentiation in mesenchymal stem cells derived from human adipose tissue. J Bone Miner Res 2009; 24: 816–825.
Cheung ST, Huang DP, Hui AB, Lo KW, Ko CW, Tsang YS et al. Nasopharyngeal carcinoma cell line (C666-1) consistently harbouring Epstein-Barr virus. Int J Cancer 1999; 83: 121–126.
Glaser R, Zhang HY, Yao KT, Zhu HC, Wang FX, Li GY et al. Two epithelial tumor cell lines (HNE-1 and HONE-1) latently infected with Epstein-Barr virus that were derived from nasopharyngeal carcinomas. Proc Natl Acad Sci USA 1989; 86: 9524–9528.
Lo AK, Liu Y, Wang XH, Huang DP, Yuen PW, Wong YC et al. Alterations of biologic properties and gene expression in nasopharyngeal epithelial cells by the Epstein-Barr virus-encoded latent membrane protein 1. Lab Invest 2003; 83: 697–709.
To EW, Chan KC, Leung SF, Chan LY, To KF, Chan AT et al. Rapid clearance of plasma Epstein-Barr virus DNA after surgical treatment of nasopharyngeal carcinoma. Clin Cancer Res 2003; 9: 3254–3259.
Shanmugaratnam K, Sobin LH . The World Health Organization histological classification of tumours of the upper respiratory tract and ear. A commentary on the second edition. Cancer 1993; 71: 2689–2697.
Feng XP, Yi H, Li MY, Li XH, Yi B, Zhang PF et al. Identification of biomarkers for predicting nasopharyngeal carcinoma response to radiotherapy by proteomics. Cancer Res 2010; 70: 3450–3462.
Acknowledgements
We thank Dr. Xiaoli Tang and Dr. Bharat Ramratnam at Warren Alpert Medical School of Brown University for kindly providing us with GFP-Drosha plasmid. This work was supported by grants from the United States Department of Defense (W81XWH-09-1-0533) and National Institute of Health (1R01CA140956, 1R21NS073098) to J-LL, and China Nature Science Foundation (81472847, 81372905) to J-LL and XPF, by Scholarships from China Scholarship Council (CSC) to XPF and XL, and an UICC YY fellowship (Feng2014/YY1/315514) to XPF.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Edited by V Stambolic
Supplementary Information accompanies this paper on Cell Death and Differentiation website
Supplementary information
Rights and permissions
About this article
Cite this article
Fang, X., Jeong, JH., Long, X. et al. IKKα-mediated biogenesis of miR-196a through interaction with Drosha regulates the sensitivity of cancer cells to radiotherapy. Cell Death Differ 23, 1471–1482 (2016). https://doi.org/10.1038/cdd.2016.32
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/cdd.2016.32