Abstract
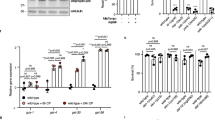
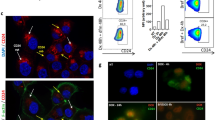
The stress-induced p38 mitogen-activated protein kinase (MAPK) pathway plays an essential role in multiple physiological processes, including cancer. In turn, p38MAPK phosphorylation at Thr180 and Tyr182 is a key regulatory mechanism for its activation and functions. Here we show that this mechanism is actively regulated through isomerisation of Pro224. Different cyclophilins can isomerise this proline residue and modulate the ability of upstream kinases to phosphorylate Thr180 and Tyr182. In vivo mutation of Pro224 to Ile in endogenous p38MAPK significantly reduced its phosphorylation and activity. This resulted in attenuation of p38MAPK signalling, which in turn caused an enhanced apoptosis and sensitivity to a DNA-damaging drug, cisplatin. We further found a reduction in size and number of lesions in homozygous mice carrying the p38MAPK P224I substitution in a K-ras model of lung tumorigenesis. We propose that cyclophilin-dependent isomerisation of p38MAPK is an important novel mechanism in regulating p38MAPK phosphorylation and functions. Thus, inhibition of this process, including with drugs that are in clinical trials, may improve the efficacy of current anti-cancer therapeutic regimes.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
Abbreviations
- MAPK:
-
mitogen-activated protein kinases
- Thr:
-
threonine
- Tyr:
-
tyrosine
- Pro:
-
proline
- Gly:
-
glycine
- UV:
-
ultraviolet
- MK2:
-
mitogene-activated protein kinase-activated protein kinase 2
- MKK:
-
matogen-activated protein kinase kinase 3
- JNK:
-
c-Jun N-terminal kinase
- Pin1:
-
peptidyl-prolyl cis–trans isomerase NIMA-interacting 1
- Cyp:
-
cyclophilin
- SILAC:
-
stable isotope labelled amino acids in cell culture
- GFP:
-
green fluorescent protein
- RNA:
-
ribonucleic acid
- DNA:
-
deoxyribonucleic acid
- Arg:
-
arginine
- TNF:
-
tumour necrosis factor
- BrdU:
-
bromodeoxyuridine
- MEF:
-
mouse embryo fibroblasts
- Cxcl5:
-
C-X-C motif chemokine 5
- PPIase:
-
peptidyl-prolyl-cis-trans-isomerase
- NF-B:
-
nuclear factor kappa-light-chain-enhancer of activated B cells
- shRNA:
-
small hairpin ribonucleic acid
References
Freshney NW, Rawlinson L, Guesdon F, Jones E, Cowley S, Hsuan J et al. Interleukin-1 activates a novel protein kinase cascade that results in the phosphorylation of Hsp27. Cell 1994; 78: 1039–1049.
Lee JC, Laydon JT, McDonnell PC, Gallagher TF, Kumar S, Green D et al. A protein kinase involved in the regulation of inflammatory cytokine biosynthesis. Nature 1994; 372: 739–746.
Bulavin DV, Higashimoto Y, Popoff IJ, Gaarde WA, Basrur V, Potapova O et al. Initiation of a G2/M checkpoint after ultraviolet radiation requires p38 kinase. Nature 2001; 411: 102–107.
Han J, Lee JD, Bibbs L, Ulevitch RJ . A MAP kinase targeted by endotoxin and hyperosmolarity in mammalian cells. Science 1994; 265: 808–811.
Reinhardt HC, Aslanian AS, Lees JA, Yaffe MB . p53-deficient cells rely on ATM- and ATR-mediated checkpoint signaling through the p38MAPK/MK2 pathway for survival after DNA damage. Cancer Cell 2007; 11: 175–189.
Bulavin DV, Demidov ON, Saito S, Kauraniemi P, Phillips C, Amundson SA et al. Amplification of PPM1D in human tumors abrogates p53 tumor-suppressor activity. Nat Genet 2002; 31: 210–215.
Hui L, Bakiri L, Mairhorfer A, Schweifer N, Haslinger C, Kenner L et al. p38alpha suppresses normal and cancer cell proliferation by antagonizing the JNK-c-Jun pathway. Nat Genet 2007; 39: 741–749.
Ventura JJ, Tenbaum S, Perdiguero E, Huth M, Guerra C, Barbacid M et al. p38alpha MAP kinase is essential in lung stem and progenitor cell proliferation and differentiation. Nat Genet 2007; 39: 750–758.
Esteva FJ, Sahin AA, Smith TL, Yang Y, Pusztai L, Nahta R et al. Prognostic significance of phosphorylated P38 mitogen-activated protein kinase and HER-2 expression in lymph node-positive breast carcinoma. Cancer 2004; 100: 499–506.
Wang SN, Lee KT, Tsai CJ, Chen YJ, Yeh YT . Phosphorylated p38 and JNK MAPK proteins in hepatocellular carcinoma. Eur J Clin Invest 2012; 42: 1295–1301.
Elenitoba-Johnson KS, Jenson SD, Abbott RT, Palais RA, Bohling SD, Lin Z et al. Involvement of multiple signaling pathways in follicular lymphoma transformation: p38-mitogen-activated protein kinase as a target for therapy. Proc Natl Acad Sci USA 2003; 100: 7259–7264.
Pomerance M, Quillard J, Chantoux F, Young J, Blondeau JP . High-level expression, activation, and subcellular localization of p38-MAP kinase in thyroid neoplasms. J Pathol 2006; 209: 298–306.
Greenberg AK, Basu S, Hu J, Yie TA, Tchou-Wong KM, Rom WN et al. Selective p38 activation in human non-small cell lung cancer. Am J Respir Cell Mol Biol 2002; 26: 558–564.
Demuth T, Reavie LB, Rennert JL, Nakada M, Nakada S, Hoelzinger DB et al. MAP-ing glioma invasion: mitogen-activated protein kinase kinase 3 and p38 drive glioma invasion and progression and predict patient survival. Mol Cancer Ther 2007; 6: 1212–1222.
Tsai MS, Weng SH, Chen HJ, Chiu YF, Huang YC, Tseng SC et al. Inhibition of p38 MAPK-dependent excision repair cross-complementing 1 expression decreases the DNA repair capacity to sensitize lung cancer cells to etoposide. Mol Cancer Ther 2012; 11: 561–571.
Germani A, Matrone A, Grossi V, Peserico A, Sanese P, Liuzzi M et al. Targeted therapy against chemoresistant colorectal cancers: inhibition of p38alpha modulates the effect of cisplatin in vitro and in vivo through the tumor suppressor FoxO3A. Cancer Lett 2014; 344: 110–118.
Pereira L, Igea A, Canovas B, Dolado I, Nebreda AR . Inhibition of p38 MAPK sensitizes tumour cells to cisplatin-induced apoptosis mediated by reactive oxygen species and JNK. EMBO Mol Med 2013; 5: 1759–1774.
Wilson KP, Fitzgibbon MJ, Caron PR, Griffith JP, Chen W, McCaffrey PG et al. Crystal structure of p38 mitogen-activated protein kinase. J Biol Chem 1996; 271: 27696–27700.
Lu KP, Finn G, Lee TH, Nicholson LK . Prolyl cis-trans isomerization as a molecular timer. Nat Chem Biol 2007; 3: 619–629.
Theuerkorn M, Fischer G, Schiene-Fischer C . Prolyl cis/trans isomerase signalling pathways in cancer. Curr Opin Pharmacol 2011; 11: 281–287.
Nelson CJ, Santos-Rosa H, Kouzarides T . Proline isomerization of histone H3 regulates lysine methylation and gene expression. Cell 2006; 126: 905–916.
Park JE, Lee JA, Park SG, Lee DH, Kim SJ, Kim HJ et al. A critical step for JNK activation: isomerization by the prolyl isomerase Pin1. Cell Death Differ 2012; 19: 153–161.
Herberich B, Cao GQ, Chakrabarti PP, Falsey JR, Pettus L, Rzasa RM et al. Discovery of highly selective and potent p38 inhibitors based on a phthalazine scaffold. J Med Chem 2008; 51: 6271–6279.
Kofron JL, Kuzmic P, Kishore V, Colon-Bonilla E, Rich DH . Determination of kinetic constants for peptidyl prolyl cis-trans isomerases by an improved spectrophotometric assay. Biochemistry 1991; 30: 6127–6134.
Lee J, Kim SS . Current implications of cyclophilins in human cancers. J Exp Clin Cancer Res 2010; 29: 97.
Choi KJ, Piao YJ, Lim MJ, Kim JH, Ha J, Choe W et al. Overexpressed cyclophilin A in cancer cells renders resistance to hypoxia- and cisplatin-induced cell death. Cancer Res 2007; 67: 3654–3662.
Galan-Moya EM, de la Cruz-Morcillo MA, Llanos Valero M, Callejas-Valera JL, Melgar-Rojas P, Hernadez Losa J et al. Balance between MKK6 and MKK3 mediates p38 MAPK associated resistance to cisplatin in NSCLC. PLoS One 2011; 6: e28406.
Johnson L, Mercer K, Greenbaum D, Bronson RT, Crowley D, Tuveson DA et al. Somatic activation of the K-ras oncogene causes early onset lung cancer in mice. Nature 2001; 410: 1111–1116.
Oliver TG, Mercer KL, Sayles LC, Burke JR, Mendus D, Lovejoy KS et al. Chronic cisplatin treatment promotes enhanced damage repair and tumor progression in a mouse model of lung cancer. Genes Dev 2010; 24: 837–852.
Yaffe MB, Schutkowski M, Shen M, Zhou XZ, Stukenberg PT, Rahfeld JU et al. Sequence-specific and phosphorylation-dependent proline isomerization: a potential mitotic regulatory mechanism. Science 1997; 278: 1957–1960.
Litchfield DW, Shilton BH, Brandl CJ, Gyenis L . Pin1: intimate involvement with the regulatory protein kinase networks in the global phosphorylation landscape. Biochim Biophys Acta 2015; 1850: 2077–2086.
Sun S, Guo M, Zhang JB, Ha A, Yokoyama KK, Chiu RH et al. (CypA) interacts with NF-kappaB subunit, p65/RelA, and contributes to NF-kappaB activation signaling. PLoS One 2014; 9: e96211.
Sun S, Wang Q, Giang A, Cheng C, Soo C, Wang CY et al. Knockdown of CypA inhibits interleukin-8 (IL-8) and IL-8-mediated proliferation and tumor growth of glioblastoma cells through down-regulated NF-kappaB. J Neurooncol 2011; 101: 1–14.
Choi JW, Schroeder MA, Sarkaria JN, Bram RJ . Cyclophilin B supports Myc and mutant p53-dependent survival of glioblastoma multiforme cells. Cancer Res 2014; 74: 484–496.
Bauer K, Kretzschmar AK, Cvijic H, Blumert C, Loffler D, Brocke-Heidrich K et al. Cyclophilins contribute to Stat3 signaling and survival of multiple myeloma cells. Oncogene 2009; 28: 2784–2795.
Lim SO, Park SJ, Kim W, Park SG, Kim HJ, Kim YI et al. Proteome analysis of hepatocellular carcinoma. Biochem Biophys Res Commun 2002; 291: 1031–1037.
Campa MJ, Wang MZ, Howard B, Fitzgerald MC, Patz EF Jr . Protein expression profiling identifies macrophage migration inhibitory factor and cyclophilin a as potential molecular targets in non-small cell lung cancer. Cancer Res 2003; 63: 1652–1656.
Li M, Zhai Q, Bharadwaj U, Wang H, Li F, Fisher WE et al. Cyclophilin A is overexpressed in human pancreatic cancer cells and stimulates cell proliferation through CD147. Cancer 2006; 106: 2284–2294.
Fang F, Flegler AJ, Du P, Lin S, Clevenger CV . Expression of cyclophilin B is associated with malignant progression and regulation of genes implicated in the pathogenesis of breast cancer. Am J Pathol 2009; 174: 297–308.
Han X, Yoon SH, Ding Y, Choi TG, Choi WJ, Kim YH et al. Cyclosporin A and sanglifehrin A enhance chemotherapeutic effect of cisplatin in C6 glioma cells. Oncol Rep 2010; 23: 1053–1062.
Li Z, Zhao X, Bai S, Wang Z, Chen L, Wei Y et al. Proteomics identification of cyclophilin a as a potential prognostic factor and therapeutic target in endometrial carcinoma. Mol Cell Proteomics 2008; 7: 1810–1823.
Al-Ghoul M, Bruck TB, Lauer-Fields JL, Asirvatham VS, Zapata C, Kerr RG et al. Comparative proteomic analysis of matched primary and metastatic melanoma cell lines. J Proteome Res 2008; 7: 4107–4118.
Wu X, Zhang W, Font-Burgada J, Palmer T, Hamil AS, Biswas SK et al. Ubiquitin-conjugating enzyme Ubc13 controls breast cancer metastasis through a TAK1-p38 MAP kinase cascade. Proc Natl Acad Sci USA 2014; 111: 13870–13875.
Li Z, Min W, Gou J . Knockdown of cyclophilin A reverses paclitaxel resistance in human endometrial cancer cells via suppression of MAPK kinase pathways. Cancer Chemother Pharmacol 2013; 72: 1001–1011.
Nakahara C, Nakamura K, Yamanaka N, Baba E, Wada M, Matsunaga H et al. Cyclosporin-A enhances docetaxel-induced apoptosis through inhibition of nuclear factor-kappaB activation in human gastric carcinoma cells. Clin Cancer Res 2003; 9: 5409–5416.
Hamilton G . Cyclophilin A as a target of Cisplatin chemosensitizers. Curr Cancer Drug Targets 2014; 14: 46–58.
Paillas S, Boissiere F, Bibeau F, Denouel A, Mollevi C, Causse A et al. Targeting the p38 MAPK pathway inhibits irinotecan resistance in colon adenocarcinoma. Cancer Res 2011; 71: 1041–1049.
Rudalska R, Dauch D, Longerich T, McJunkin K, Wuestefeld T, Kang TW et al. In vivo RNAi screening identifies a mechanism of sorafenib resistance in liver cancer. Nat Med 2014; 20: 1138–1146.
Morandell S, Reinhardt HC, Cannell IG, Kim JS, Ruf DM, Mitra T et al. A reversible gene-targeting strategy identifies synthetic lethal interactions between MK2 and p53 in the DNA damage response in vivo. Cell Rep 2013; 5: 868–877.
Wong ES, Le Guezennec X, Demidov ON, Marshall NT, Wang ST, Krishnamurthy J et al. p38MAPK controls expression of multiple cell cycle inhibitors and islet proliferation with advancing age. Dev Cell 2009; 17: 142–149.
Acknowledgements
The research of DVB was supported by the Foundation ARC (France) and for AB by A*STAR’s JCO project grant 14302FG090 (Singapore). We are grateful to Dr. Elise Courtois, Jun Siong Low, Nancy Zhao Qi and Matthias Schmitt for the assistance.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Edited by P Salomoni
Supplementary Information accompanies this paper on Cell Death and Differentiation website
Supplementary information
Rights and permissions
About this article
Cite this article
Brichkina, A., Nguyen, N., Baskar, R. et al. Proline isomerisation as a novel regulatory mechanism for p38MAPK activation and functions. Cell Death Differ 23, 1592–1601 (2016). https://doi.org/10.1038/cdd.2016.45
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/cdd.2016.45
This article is cited by
-
Diversity and versatility of p38 kinase signalling in health and disease
Nature Reviews Molecular Cell Biology (2021)
-
Connexin43 dephosphorylation at serine 282 is associated with connexin43-mediated cardiomyocyte apoptosis
Cell Death & Differentiation (2019)