Abstract
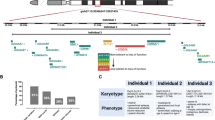
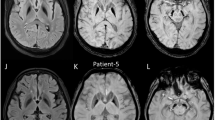
Chromosomal abnormalities are implicated in a substantial number of human developmental syndromes, but for many such disorders little is known about the causative genes. The recently described 1q41q42 microdeletion syndrome is characterized by characteristic dysmorphic features, intellectual disability and brain morphological abnormalities, but the precise genetic basis for these abnormalities remains unknown. Here, our detailed analysis of the genetic abnormalities of 1q41q42 microdeletion cases identified TP53BP2, which encodes apoptosis-stimulating protein of p53 2 (ASPP2), as a candidate gene for brain abnormalities. Consistent with this, Trp53bp2-deficient mice show dilation of lateral ventricles resembling the phenotype of 1q41q42 microdeletion patients. Trp53bp2 deficiency causes 100% neonatal lethality in the C57BL/6 background associated with a high incidence of neural tube defects and a range of developmental abnormalities such as congenital heart defects, coloboma, microphthalmia, urogenital and craniofacial abnormalities. Interestingly, abnormalities show a high degree of overlap with 1q41q42 microdeletion-associated abnormalities. These findings identify TP53BP2 as a strong candidate causative gene for central nervous system (CNS) defects in 1q41q42 microdeletion syndrome, and open new avenues for investigation of the mechanisms underlying CNS abnormalities.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
Abbreviations
- ASPP2:
-
apoptosis-stimulating protein of p53, 2
- NTD:
-
neural tube defect
- CNS:
-
central nervous system
- CAPN2:
-
calpain 2
- CAPN8:
-
calpain 8
- FBXO28:
-
F-box only protein 28
- SRO:
-
smallest region of overlap
- MRI:
-
magnetic resonance imaging
- LV:
-
lateral ventricle
- NIH:
-
National Institutes of Health
- microCT:
-
micro-computed tomography
- HREM:
-
high-resolution episcopic microscopy
- B6:
-
C57BL6/J
- VSD:
-
ventricular septal defect
- IMPC:
-
International Mouse Phenotyping Consortium
- CHARGE:
-
Coloboma, Heart defects, Atresia of the choanae, Retardation of growth and/or development, Genital and/or urinary abnormalities, Ear abnormalities
- EMT:
-
epithelial-to-mesenchymal transition
References
Tyshchenko N, Lurie I, Schinzel A . Chromosomal map of human brain malformations. Hum Genet 2008; 124: 73–80.
Brewer C, Holloway S, Zawalnyski P, Schinzel A, FitzPatrick D . A chromosomal deletion map of human malformations. Am J Hum Genet 1998; 63: 1153–1159.
Rosenfeld JA, Lacassie Y, El-Khechen D, Escobar LF, Reggin J, Heuer C et al. New cases and refinement of the critical region in the 1q41q42 microdeletion syndrome. Eur J Med Genet 2011; 54: 42–49.
Au PYB, Argiropoulos B, Parboosingh JS, Micheil Innes A . Refinement of the critical region of 1q41q42 microdeletion syndrome identifies FBXO28 as a candidate causative gene for intellectual disability and seizures. Am J Med Genet A 2014; 164: 441–448.
Cepeda D, Ng HF, Sharifi HR, Mahmoudi S, Cerrato VS, Fredlund E et al. CDK‐mediated activation of the SCFFBXO28 ubiquitin ligase promotes MYC‐driven transcription and tumourigenesis and predicts poor survival in breast cancer. EMBO Molec Med 2013; 5: 1067–1086.
Gorina S, Pavletich NP . Structure of the p53 tumor suppressor bound to the Ankyrin and SH3 domains of 53BP2. Science 1996; 274: 1001–1005.
Samuels-Lev Y, O'Connor DJ, Bergamaschi D, Trigiante G, Hsieh J-K, Zhong S et al. ASPP proteins specifically stimulate the apoptotic function of p53. Mol Cell 2001; 8: 781–794.
Kampa KM, Acoba JD, Chen D, Gay J, Lee H, Beemer K et al. Apoptosis-stimulating protein of p53 (ASPP2) heterozygous mice are tumor-prone and have attenuated cellular damage–response thresholds. Proc Natl Acad Sci USA 2009; 106: 4390–4395.
Vives V, Su J, Zhong S, Ratnayaka I, Slee E, Goldin R et al. ASPP2 is a haploinsufficient tumor suppressor that cooperates with p53 to suppress tumor growth. Gene Dev 2006; 20: 1262–1267.
Sottocornola R, Royer C, Vives V, Tordella L, Zhong S, Wang Y et al. ASPP2 binds Par-3 and controls the polarity and proliferation of neural progenitors during CNS development. Dev Cell 2010; 19: 126–137.
Bergamaschi D, Samuels Y, Jin B, Duraisingham S, Crook T, Lu X . ASPP1 and ASPP2: common activators of p53 family members. Mol Cell Biol 2004; 24: 1341–1350.
Gao Y, Chen X, Shangguan S, Bao Y, Lu X, Zou J et al. Association study of PARD3 gene polymorphisms with neural tube defects in a Chinese Han population. Reprod Sci 2012; 19: 764–771.
Armstrong JF, Kaufman MH, Harrison DJ, Clarke AR . High-frequency developmental abnormalities in p53-deficient mice. Curr Biol 1995; 5: 931–936.
Sah VP, Attardi LD, Mulligan GJ, Williams BO, Bronson RT, Jacks T . A subset of p53-deficient embryos exhibit exencephaly. Nat Genet 1995; 10: 175–180.
Gu X, Xing L, Shi G, Liu Z, Wang X, Qu Z et al. The circadian mutation PER2(S662G) is linked to cell cycle progression and tumorigenesis. Cell Death Differ 2012; 19: 397–405.
Eom DS, Amarnath S, Agarwala S . Apicobasal polarity and neural tube closure. Dev Growth Differ 2013; 55: 164–172.
Monier B, Gettings M, Gay G, Mangeat T, Schott S, Guarner A et al. Apico-basal forces exerted by apoptotic cells drive epithelium folding. Nature 2015; 518: 245–248.
Yamaguchi Y, Shinotsuka N, Nonomura K, Takemoto K, Kuida K, Yosida H et al. Live imaging of apoptosis in a novel transgenic mouse highlights its role in neural tube closure. J Cell Biol 2011; 195: 1047–1060.
Shaffer LG, Theisen A, Bejjani BA, Ballif BC, Aylsworth AS, Lim C et al. The discovery of microdeletion syndromes in the post-genomic era: review of the methodology and characterization of a new 1q41q42 microdeletion syndrome. Genet Med 2007; 9: 607–616.
Mazzeu JF, Krepischi-Santos AC, Rosenberg C, Szuhai K, Knijnenburg J, Weiss JMM et al. Chromosome abnormalities in two patients with features of autosomal dominant Robinow syndrome. Am J Med Genet A 2007; 143A: 1790–1795.
Mazzeu JF, Vianna-Morgante AM, Krepischi ACV, Oudakker A, Rosenberg C, Szuhai K et al. Deletions encompassing 1q41q42.1 and clinical features of autosomal dominant Robinow syndrome. Clin Genet 2010; 77: 404–407.
Rice GM, Qi Z, Selzer R, Richmond T, Thompson K, Pauli RM et al. Microdissection-based high-resolution genomic array analysis of two patients with cytogenetically identical interstitial deletions of chromosome 1q but distinct clinical phenotypes. Am J Med Genet A 2006; 140 A: 1637–1643.
Filges I, Röthlisberger B, Boesch N, Weber P, Wenzel F, Huber AR et al. Interstitial deletion 1q42 in a patient with agenesis of corpus callosum: phenotype–genotype comparison to the 1q41q42 microdeletion suggests a contiguous 1q4 syndrome. Am J Med Genet A 2010; 152 A: 987–993.
Spreiz A, Haberlandt E, Baumann M, Baumgartner Sigl S, Fauth C, Gautsch K et al. Chromosomal microaberrations in patients with epilepsy, intellectual disability, and congenital anomalies. Clin Genet 2014; 86: 361–366.
Christensen RD, Yaish HM . A neonate with the Pelger-Huet anomaly, cleft lip and palate, and agenesis of the corpus callosum, with a chromosomal microdeletion involving 1q41 to 1q42.12. J Perinatol 2012; 32: 238–240.
Wat MJ, Veenma D, Hogue J, Holder AM, Yu Z, Wat JJ et al. Genomic alterations that contribute to the development of isolated and non-isolated congenital diaphragmatic hernia. J Med Genet 2011; 48: 299–307.
Ono Y, Sorimachi H . Calpains — an elaborate proteolytic system. Biochim Biophys Acta 2012; 1824: 224–236.
Takano J, Mihira N, Fujioka R, Hosoki E, Chishti AH, Saido TC . Vital role of the calpain-calpastatin system for placental-integrity-dependent embryonic survival. Mol Cell Biol 2011; 31: 4097–4106.
Dutt P, Croall D, Arthur JS, Veyra TD, Williams K, Elce J et al. m-Calpain is required for preimplantation embryonic development in mice. BMC Dev Biol 2006; 6: 3.
Hata S, Abe M, Suzuki H, Kitamura F, Toyama-Sorimachi N, Abe K et al. Calpain 8/nCL-2 and Calpain 9/nCL-4 constitute an active protease complex, G-calpain, involved in gastric mucosal defense. PLoS Genet 2010; 6: e1001040.
Lonsdale J, Thomas J, Salvatore M, Phillips R, Lo E, Shad S et al. The Genotype-Tissue Expression (GTEx) project. Nat Genet 2013; 45: 580–585.
Hawrylycz M, Ng L, Feng D, Sunkin S, Szafer A, Dang C. The Allen Brain Atlas. In: Kasabov N (ed.). Springer Handbook of Bio-/Neuroinformatics. Springer: Berlin, Heidelberg, 2014, pp. 1111–1126.
Miller JA, Ding S-L, Sunkin SM, Smith KA, Ng L, Szafer A et al. Transcriptional landscape of the prenatal human brain. Nature 2014; 508: 199–206.
Coe BP, Witherspoon K, Rosenfeld JA, van Bon BWM, Vulto-van Silfhout AT, Bosco P et al. Refining analyses of copy number variation identifies specific genes associated with developmental delay. Nat Genet 2014; 46: 1063–1071.
Cooper GM, Coe BP, Girirajan S, Rosenfeld JA, Vu TH, Baker C et al. A copy number variation morbidity map of developmental delay. Nat Genet 2011; 43: 838–846.
Shaikh TH, Gai X, Perin JC, Glessner JT, Xie H, Murphy K et al. High-resolution mapping and analysis of copy number variations in the human genome: a data resource for clinical and research applications. Genome Res 2009; 19: 1682–1690.
Weninger WJ, Meng S, Streicher J, Mueller GB . A new episcopic method for rapid 3-D reconstruction: applications in anatomy and embryology. Anat Embryol 1998; 197: 341–348.
Rowland CA, Correa A, Cragan JD, Alverson CJ . Are Encephaloceles neural tube defects? Pediatrics 2006; 118: 916–923.
National Guideline CNeural Tube Defects. Agency for Healthcare Research and Quality (AHRQ): Rockville, MD, USA, 2013.
Brown SDM, Moore MW . The International Mouse Phenotyping Consortium: past and future perspectives on mouse phenotyping. Mammalian Genome 2012; 23: 632–640.
Cassina M, Rigon C, Casarin A, Vicenzi V, Salviati L, Clementi M . FBXO28 is a critical gene of the 1q41q42 microdeletion syndrome. Am J Med Genet A 2015; 167: 1418–1420.
Jun KR, Hur YJ, Lee JN, Kim HR, Shin JH, Oh SH et al. Clinical characterization of DISP1 haploinsufficiency: a case report. Eur J Med Genet 2013; 56: 309–313.
The International Mouse Knockout C. A mouse for all reasons. Cell 2007; 128: 9–13.
Van Nostrand JL, Brady CA, Jung H, Fuentes DR, Kozak MM, Johnson TM et al. Inappropriate p53 activation during development induces features of CHARGE syndrome. Nature 2014; 514: 228–232.
Sanlaville D, Verloes A . CHARGE syndrome: an update. Eur J Hum Genet 2007; 15: 389–399.
Wang Y, Bu F, Royer C, Serres S, Larkin JR, Soto Manuel S et al. ASPP2 controls epithelial plasticity and inhibits metastasis through β-catenin-dependent regulation of ZEB1. Nat Cell Biol 2014; 16: 1092–1104.
Ray HJ, Niswander LA . Grainyhead-like 2 downstream targets act to suppress EMT during neural tube closure. Development 2016; 143: 1192–1204.
Kempton MJ, Underwood TSA, Brunton S, Stylios F, Schmechtig A, Ettinger U et al. A comprehensive testing protocol for MRI neuroanatomical segmentation techniques: evaluation of a novel lateral ventricle segmentation method. NeuroImage 2011; 58: 1051–1059.
Mohun TJ, Leong LM, Weninger WJ, Sparrow DB . The morphology of heart development in Xenopus laevis. Dev Biol 2000; 218: 74–88.
Kent WJ, Sugnet CW, Furey TS, Roskin KM, Pringle TH, Zahler AM et al. The Human Genome Browser at UCSC. Genome Res 2002; 12: 996–1006.
Wolfe D, Dudek S, Ritchie M, Pendergrass S . Visualizing genomic information across chromosomes with PhenoGram. BioData Min 2013; 6: 18.
Team RDC R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing: Vienna, Austria, 2008.
Acknowledgements
This work was funded by the Ludwig Institute for Cancer Research. JZ is supported by the Skaggs-Oxford Scholarship (The Scripps Research Institute). JES acknowledges support from the BHF (FS/11/50/29038), and the BHF Centre of Research Excellence, Oxford (RE/08/004). We thank Mary Muers and Andrew Wilkie for critical reading of the manuscript, and Michael Schocke for radiological assistance. This study makes use of data generated by the DECIPHER community. A full list of centres who contributed to the generation of the data is available from http://decipher.sanger.ac.uk and via email from decipher@sanger.ac.uk. Funding for the DECIPHER project was provided by the Wellcome Trust. Normal MRI controls used in the preparation of this article were obtained from the Pediatric MRI Data Repository. This is a multi-site, longitudinal study of typically developing children, from ages newborn through young adulthood, conducted by the Brain Development Cooperative Group and supported by the National Institute of Child Health and Human Development, the National Institute on Drug Abuse, the National Institute of Mental Health, and the National Institute of Neurological Disorders and Stroke (Contract Nos. N01-HD02-3343, N01-MH9-0002, and N01-NS-9-2314, -2315, -2316, -2317, -2319 and -2320). A listing of the participating sites and a complete listing of the study investigators can be found at http://www.bic.mni.mcgill.ca/nihpd/info/participating_centers.html.
Author contributions
JZ and XL designed the study; JZ, VV, DS, AV, JES, PM, ES, FP, CAL, and TJM performed the experiments; SJ, YL, EC, LFE, MT, ASA, HD, TAC, JA, ADC, EH, DK, DAS, MJP, ZZ, YSC, DW, AMI, KRJ, and SZ contributed clinical data; JZ, PP, and JAR analysed clinical data, JZ, VV, and XL wrote the paper; all authors analysed the data and reviewed the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Edited by G Melino
Disclaimer
This manuscript reflects the views of the authors and may not reflect the opinions or views of the NIH.
Supplementary Information accompanies this paper on Cell Death and Differentiation website
Supplementary information
Rights and permissions
About this article
Cite this article
Zak, J., Vives, V., Szumska, D. et al. ASPP2 deficiency causes features of 1q41q42 microdeletion syndrome. Cell Death Differ 23, 1973–1984 (2016). https://doi.org/10.1038/cdd.2016.76
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/cdd.2016.76