Abstract
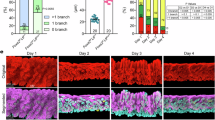
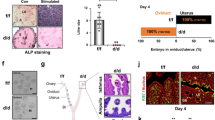
Formation of secretary endometrial glands in the uterus known as adenogenesis is a typical process of branching morphogenesis involving dynamic epithelial growth and differentiation. Unsuccessful adenogenesis often leads to female infertility. However, it remains largely unexplored so far regarding the epigenetic machinery governing normal endometrial gland formation. Here, we demonstrated that PR-Set7, an epigenetic regulator for H4K20me1 modification, was extensively expressed in the postnatal uteri, and its conditional deletion resulted in a complete lack of endometrial glands and infertility in mice. Subsequent analysis revealed that uterine PR-Set7 deficiency abolishes the dynamic endometrial epithelial population growth during the short span of gland formation from postnatal days 3 to 9. This markedly reduced epithelial population growth in PR-Set7-null mutant uteri is well associated with DNA damage accumulation and massive apoptotic death in the epithelium, due to blockade of 53BP1 recruitment to DNA damage sites upon reduced levels of H4K20me1/2. Using PgrCre/+/Rosa26DTA/+ mouse line and postnatal progesterone injection mouse model, we further confirmed that an impaired epithelial cell population growth either by inducing epithelial death in the diphtheria toxin-A (DTA)-mouse model or attenuating epithelial growth upon postnatal progesterone treatment similarly hampers uterine adenogenesis. Collectively, we establish here a novel ‘epithelial population growth threshold’ model for successful gland development. Besides further shedding light on the regulatory machinery governing uterine gland formation, our findings raise a safety concern on progesterone supplementation to prevent preterm birth in women bearing a female fetus, as exogenous progesterone may hamper uterine adenogenesis via attenuating epithelial population growth.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Gray CA, Bartol FF, Tarleton BJ, Wiley AA, Johnson GA, Bazer FW et al. Developmental biology of uterine glands. Biol Reprod 2001; 65: 1311–1323.
Spencer TE, Hayashi K, Hu J, Carpenter KD . Comparative developmental biology of the mammalian uterus. Curr Top Dev Biol 2005; 68: 85–122.
Brody JR, Cunha GR . Histologic, morphometric, and immunocytochemical analysis of myometrial development in rats and mice: II. Effects of DES on development. Am J Anat 1989; 186: 21–42.
Jeong JW, Kwak I, Lee KY, Kim TH, Large MJ, Stewart CL et al. Foxa2 is essential for mouse endometrial gland development and fertility. Biol Reprod 2010; 83: 396–403.
Filant J, Spencer TE . Endometrial glands are essential for blastocyst implantation and decidualization in the mouse uterus. Biol Reprod 2013; 88: 93.
Spencer TE . Biological roles of uterine glands in pregnancy. Semin Reprod Med 2014; 32: 346–357.
Kobayashi A, Behringer RR . Developmental genetics of the female reproductive tract in mammals. Nat Rev Genet 2003; 4: 969–980.
Bellessort B, Le Cardinal M, Bachelot A, Narboux-Neme N, Garagnani P, Pirazzini C et al. Dlx5 and Dlx6 control uterine adenogenesis during post-natal maturation: possible consequences for endometriosis. Hum Mol Genet 2016; 25: 97–108.
Kim YS, Kim HR, Kim H, Yang SC, Park M, Yoon JA et al. Deficiency in DGCR8-dependent canonical microRNAs causes infertility due to multiple abnormalities during uterine development in mice. Sci Rep 2016; 6: 20242.
Dunlap KA, Filant J, Hayashi K, Rucker EB 3rd, Song G, Deng JM et al. Postnatal deletion of Wnt7a inhibits uterine gland morphogenesis and compromises adult fertility in mice. Biol Reprod 2011; 85: 386–396.
Cooke PS, Spencer TE, Bartol FF, Hayashi K . Uterine glands: development, function and experimental model systems. Mol Hum Reprod 2013; 19: 547–558.
Farah O, Biechele S, Rossant J, Dufort D . Porcupine-dependent Wnt signaling controls stromal proliferation and endometrial gland maintenance through the action of distinct WNTs. Dev Biol 2016; 422: 58–69.
Kelleher AM, Peng W, Pru JK, Pru CA, DeMayo FJ, Spencer TE . Forkhead box a2 (FOXA2) is essential for uterine function and fertility. Proc Natl Acad Sci USA 2017; 114: E1018–E1026.
Xiao B, Jing C, Kelly G, Walker PA, Muskett FW, Frenkiel TA et al. Specificity and mechanism of the histone methyltransferase Pr-Set7. Genes Dev 2005; 19: 1444–1454.
Nishioka K, Rice JC, Sarma K, Erdjument-Bromage H, Werner J, Wang Y et al. PR-Set7 is a nucleosome-specific methyltransferase that modifies lysine 20 of histone H4 and is associated with silent chromatin. Mol Cell 2002; 9: 1201–1213.
Beck DB, Oda H, Shen SS, Reinberg D . PR-Set7 and H4K20me1: at the crossroads of genome integrity, cell cycle, chromosome condensation, and transcription. Genes Dev 2012; 26: 325–337.
Oda H, Okamoto I, Murphy N, Chu J, Price SM, Shen MM et al. Monomethylation of histone H4-lysine 20 is involved in chromosome structure and stability and is essential for mouse development. Mol Cell Biol 2009; 29: 2278–2295.
Driskell I, Oda H, Blanco S, Nascimento E, Humphreys P, Frye M . The histone methyltransferase Setd8 acts in concert with c-Myc and is required to maintain skin. EMBO J 2012; 31: 616–629.
Nikolaou KC, Moulos P, Chalepakis G, Hatzis P, Oda H, Reinberg D et al. Spontaneous development of hepatocellular carcinoma with cancer stem cell properties in PR-SET7-deficient livers. EMBO J 2015; 34: 430–447.
Dulev S, Tkach J, Lin S, Batada NN . SET8 methyltransferase activity during the DNA double-strand break response is required for recruitment of 53BP1. EMBO Rep 2014; 15: 1163–1174.
Tuzon CT, Spektor T, Kong X, Congdon LM, Wu S, Schotta G et al. Concerted activities of distinct H4K20 methyltransferases at DNA double-strand breaks regulate 53BP1 nucleation and NHEJ-directed repair. Cell Rep 2014; 8: 430–438.
Dantuma NP, van Attikum H . Spatiotemporal regulation of posttranslational modifications in the DNA damage response. EMBO J 2016; 35: 6–23.
Baldock RA, Day M, Wilkinson OJ, Cloney R, Jeggo PA, Oliver AW et al. ATM localization and heterochromatin repair depend on direct interaction of the 53BP1-BRCT2 domain with gammaH2AX. Cell Rep 2015; 13: 2081–2089.
Allison GC, Bartol FF, Taylor KM, Wiley AA, Ramsey WS, Ott TL et al. Ovine uterine gland knock-out model: effects of gland ablation on the estrous cycle. Biol Reprod 2000; 62: 448–456.
Gray CA, Burghardt RC, Johnson GA, Bazer FW, Spencer TE . Evidence that absence of endometrial gland secretions in uterine gland knockout ewes compromises conceptus survival and elongation. Reproduction 2002; 124: 289–300.
Cooke PS, Ekman GC, Kaur J, Davila J, Bagchi IC, Clark SG et al. Brief exposure to progesterone during a critical neonatal window prevents uterine gland formation in mice. Biol Reprod 2012; 86: 63.
Zhang S, Lin H, Kong S, Wang S, Wang H, Wang H et al. Physiological and molecular determinants of embryo implantation. Mol Aspects Med 2013; 34: 939–980.
Affolter M, Bellusci S, Itoh N, Shilo B, Thiery JP, Werb Z . Tube or not tube: remodeling epithelial tissues by branching morphogenesis. Dev Cell 2003; 4: 11–18.
Norwitz ER, Caughey AB . Progesterone supplementation and the prevention of preterm birth. Rev Obstet Gynecol 2011; 4: 60–72.
Navathe R, Berghella V . Progesterone as a tocolytic agent for preterm labor: a systematic review. Curr Opin Obstet Gynecol 2016; 28: 464–469.
Fonseca EB, Celik E, Parra M, Singh M, Nicolaides KH . Progesterone and the risk of preterm birth among women with a short cervix. N Engl J Med 2007; 357: 462–469.
Iams JD, Romero R, Culhane JF, Goldenberg RL . Primary, secondary, and tertiary interventions to reduce the morbidity and mortality of preterm birth. Lancet 2008; 371: 164–175.
Norman JE, Marlow N, Messow C-M, Shennan A, Bennett PR, Thornton S et al. Vaginal progesterone prophylaxis for preterm birth (the OPPTIMUM study): a multicentre, randomised, double-blind trial. Lancet 2016; 387: 2106–2116.
Ivanova A, Signore M, Caro N, Greene ND, Copp AJ, Martinez-Barbera JP . In vivo genetic ablation by Cre-mediated expression of diphtheria toxin fragment A. Genesis 2005; 43: 129–135.
Soyal SM, Mukherjee A, Lee KY, Li J, Li H, DeMayo FJ et al. Cre-mediated recombination in cell lineages that express the progesterone receptor. Genesis 2005; 41: 58–66.
Lu J, Zhang S, Nakano H, Simmons DG, Wang S, Kong S et al. A positive feedback loop involving Gcm1 and Fzd5 directs chorionic branching morphogenesis in the placenta. PLoS Biol 2013; 11: e1001536.
Wang Q, Lu J, Zhang S, Wang S, Wang W, Wang B et al. Wnt6 is essential for stromal cell proliferation during decidualization in mice. Biol Reprod 2013; 88: 5.
Acknowledgements
We are grateful to Dr. Francesco DeMayo (National Institute of Environmental Health Sciences, USA) for his generosity in providing us with the PgrCre/+ mice. This work was supported by National Key R&D Program of China (2017YFC1001402 to HW, 2017YF0104603 to SK and JL) and the National Natural Science Foundation (81330017 and 81490744 to HW, 81601285 to SK and 31600945 to JL).
Author contributions
WH and LJ designed research; CT, HB, KS, ZC, NZ and BH performed research; WH, LJ, CT, ZH, QJ and XQ analyzed the data; DR and JPL provided mouse models; CT, LJ and WH wrote the paper.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Edited by P Salomoni
Supplementary Information accompanies this paper on Cell Death and Differentiation website
Supplementary information
Rights and permissions
About this article
Cite this article
Cui, T., He, B., Kong, S. et al. PR-Set7 deficiency limits uterine epithelial population growth hampering postnatal gland formation in mice. Cell Death Differ 24, 2013–2021 (2017). https://doi.org/10.1038/cdd.2017.120
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/cdd.2017.120
This article is cited by
-
DCAF13 is essential for mouse uterine function and fertility
Cell Death Discovery (2025)
-
Alterations in the Hippo Signaling Pathway During Adenogenesis Impairment in Postnatal Mouse Uterus
Reproductive Sciences (2025)
-
PR-SET7 epigenetically restrains uterine interferon response and cell death governing proper postnatal stromal development
Nature Communications (2024)
-
De novo reconstruction of a functional in vivo-like equine endometrium using collagen-based tissue engineering
Scientific Reports (2024)
-
SETD8 inhibition targets cancer cells with increased rates of ribosome biogenesis
Cell Death & Disease (2024)