Abstract
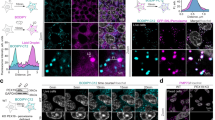
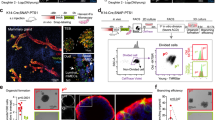
A recent report from the Laboratory of Heidi McBride (McGill University) presents a role for mitochondria in the de novo biogenesis of peroxisomes in mammalian cells. Peroxisomes are essential organelles responsible for a wide variety of biochemical functions, from the generation of bile to plasmalogen synthesis, reduction of peroxides, and the oxidation of very-long-chain fatty acids. Like mitochondria, peroxisomes proliferate primarily through growth and division of pre-existing peroxisomes. However, unlike mitochondria, peroxisomes do not fuse; further, and perhaps most importantly, they can also be born de novo, a process thought to occur through the generation of pre-peroxisomal vesicles that originate from the endoplasmic reticulum. De novo peroxisome biogenesis has been extensively studied in yeast, with a major focus on the role of the ER in this process; however, in the mammalian system this field is much less explored. By exploiting patient cells lacking mature peroxisomes, the McBride laboratory now assigns a role to ER and mitochondria in de novo mammalian peroxisome biogenesis by showing that the formation of immature pre-peroxisomes occurs through the fusion of Pex3-/Pex14-containing mitochondria-derived vesicles with Pex16-containing ER-derived vesicles.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Sugiura A, Mattie S, Prudent J, McBride HM . Newly born peroxisomes are a hybrid of mitochondrial and ER-derived pre-peroxisomes. Nature 2017; 542: 251–254.
Wanders RJ . Peroxisomes in human health and disease: metabolic pathways, metabolite transport, interplay with other organelles and signal transduction. Subcell Biochem 2013; 69: 23–44.
Lazarow PB, Fujiki Y . Biogenesis of peroxisomes. Annu Rev Cell Biol 1985; 1: 489–530.
Motley AM, Hettema EH . Yeast peroxisomes multiply by growth and division. J Cell Biol 2007; 178: 399–410.
Huybrechts SJ, Van Veldhoven PP, Brees C, Mannaerts GP, Los GV, Fransen M . Peroxisome dynamics in cultured mammalian cells. Traffic 2009; 10: 1722–1733.
Schrader M, Costello JL, Godinho LF, Azadi AS, Islinger M . Proliferation and fission of peroxisomes - an update. Biochim Biophys Acta 2016; 1863: 971–983.
Bonekamp NA, Sampaio P, de Abreu FV, Luers GH, Schrader M . Transient complex interactions of mammalian peroxisomes without exchange of matrix or membrane marker proteins. Traffic 2012; 13: 960–978.
Agrawal G, Subramani S . De novo peroxisome biogenesis: evolving concepts and conundrums. Biochim Biophys Acta 2016; 1863: 892–901.
Hua R, Kim PK . Multiple paths to peroxisomes: mechanism of peroxisome maintenance in mammals. Biochim Biophys Acta 2016; 1863: 881–891.
Kim PK, Mullen RT, Schumann U, Lippincott-Schwartz J . The origin and maintenance of mammalian peroxisomes involves a de novo PEX16-dependent pathway from the ER. J Cell Biol 2006; 173: 521–532.
Delille HK, Agricola B, Guimaraes SC, Borta H, Luers GH, Fransen M et al. Pex11pbeta-mediated growth and division of mammalian peroxisomes follows a maturation pathway. J Cell Sci 2010; 123: 2750–2762.
Koch J, Pranjic K, Huber A, Ellinger A, Hartig A, Kragler F et al. PEX11 family members are membrane elongation factors that coordinate peroxisome proliferation and maintenance. J Cell Sci 2010; 123: 3389–3400.
Smith JJ, Aitchison JD . Peroxisomes take shape. Nat Rev Mol Cell Biol 2013; 14: 803–817.
Yuan W, Veenhuis M, van der Klei IJ . The birth of yeast peroxisomes. Biochim Biophys Acta 2016; 1863: 902–910.
van der Zand A, Gent J, Braakman I, Tabak HF . Biochemically distinct vesicles from the endoplasmic reticulum fuse to form peroxisomes. Cell 2012; 149: 397–409.
Knoops K, Manivannan S, Cepinska MN, Krikken AM, Kram AM, Veenhuis M et al. Preperoxisomal vesicles can form in the absence of Pex3. J Cell Biol 2014; 204: 659–668.
Motley AM, Galvin PC, Ekal L, Nuttall JM, Hettema EH . Reevaluation of the role of Pex1 and dynamin-related proteins in peroxisome membrane biogenesis. J Cell Biol 2015; 211: 1041–1056.
Hettema EH, Erdmann R, van der Klei I, Veenhuis M . Evolving models for peroxisome biogenesis. Curr Opin Cell Biol 2014; 29: 25–30.
Honsho M, Tamura S, Shimozawa N, Suzuki Y, Kondo N, Fujiki Y . Mutation in PEX16 is causal in the peroxisome-deficient Zellweger syndrome of complementation group D. Am J Hum Genet 1998; 63: 1622–1630.
Kiel JA, Veenhuis M, van der Klei IJ . PEX genes in fungal genomes: common, rare or redundant. Traffic 2006; 7: 1291–1303.
Matsuzaki T, Fujiki Y . The peroxisomal membrane protein import receptor Pex3p is directly transported to peroxisomes by a novel Pex19p- and Pex16p-dependent pathway. J Cell Biol 2008; 183: 1275–1286.
Muntau AC, Mayerhofer PU, Paton BC, Kammerer S, Roscher AA . Defective peroxisome membrane synthesis due to mutations in human PEX3 causes Zellweger syndrome, complementation group G. Am J Hum Genet 2000; 67: 967–975.
Hoepfner D, Schildknegt D, Braakman I, Philippsen P, Tabak HF . Contribution of the endoplasmic reticulum to peroxisome formation. Cell 2005; 122: 85–95.
Sacksteder KA, Jones JM, South ST, Li X, Liu Y, Gould SJ . PEX19 binds multiple peroxisomal membrane proteins, is predominantly cytoplasmic, and is required for peroxisome membrane synthesis. J Cell Biol 2000; 148: 931–944.
Neuspiel M, Schauss AC, Braschi E, Zunino R, Rippstein P, Rachubinski RA et al. Cargo-selected transport from the mitochondria to peroxisomes is mediated by vesicular carriers. Curr Biol 2008; 18: 102–108.
Goldfischer S, Moore CL, Johnson AB, Spiro AJ, Valsamis MP, Wisniewski HK et al. Peroxisomal and mitochondrial defects in the cerebro-hepato-renal syndrome. Science 1973; 182: 62–64.
Klouwer FC, Berendse K, Ferdinandusse S, Wanders RJ, Engelen M, Poll-The BT . Zellweger spectrum disorders: clinical overview and management approach. Orphanet J Rare Dis 2015; 10: 151.
Gould SJ, Valle D . Peroxisome biogenesis disorders: genetics and cell biology. Trends Genet 2000; 16: 340–345.
Erdmann R . Assembly, maintenance and dynamics of peroxisomes. Biochim Biophys Acta 2016; 1863: 787–789.
Soubannier V, McLelland GL, Zunino R, Braschi E, Rippstein P, Fon EA et al. A vesicular transport pathway shuttles cargo from mitochondria to lysosomes. Curr Biol 2012; 22: 135–141.
Rucktaschel R, Halbach A, Girzalsky W, Rottensteiner H, Erdmann R . De novo synthesis of peroxisomes upon mitochondrial targeting of Pex3p. Eur J Cell Biol 2010; 89: 947–954.
Yamashita S, Abe K, Tatemichi Y, Fujiki Y . The membrane peroxin PEX3 induces peroxisome-ubiquitination-linked pexophagy. Autophagy 2014; 10: 1549–1564.
Knoblach B, Rachubinski RA . How peroxisomes partition between cells. A story of yeast, mammals and filamentous fungi. Curr Opin Cell Biol 2016; 41: 73–80.
Williams C, van der Klei IJ . Pexophagy-linked degradation of the peroxisomal membrane protein Pex3p involves the ubiquitin-proteasome system. Biochem Biophys Res Commun 2013; 438: 395–401.
Platta HW, El Magraoui F, Schlee D, Grunau S, Girzalsky W, Erdmann R . Ubiquitination of the peroxisomal import receptor Pex5p is required for its recycling. J Cell Biol 2007; 177: 197–204.
Braschi E, Goyon V, Zunino R, Mohanty A, Xu L, McBride HM . Vps35 mediates vesicle transport between the mitochondria and peroxisomes. Curr Biol 2010; 20: 1310–1315.
Sugiura A, McLelland GL, Fon EA, McBride HM . A new pathway for mitochondrial quality control: mitochondrial-derived vesicles. EMBO J 2014; 33: 2142–2156.
McLelland GL, Soubannier V, Chen CX, McBride HM, Fon EA . Parkin and PINK1 function in a vesicular trafficking pathway regulating mitochondrial quality control. EMBO J 2014; 33: 282–295.
McLelland GL, Lee SA, McBride HM, Fon EA . Syntaxin-17 delivers PINK1/parkin-dependent mitochondrial vesicles to the endolysosomal system. J Cell Biol 2016; 214: 275–291.
Matheoud D, Sugiura A, Bellemare-Pelletier A, Laplante A, Rondeau C, Chemali M et al. Parkinson's disease-related proteins PINK1 and parkin repress mitochondrial antigen presentation. Cell 2016; 166: 314–327.
Caielli S, Athale S, Domic B, Murat E, Chandra M, Banchereau R et al. Oxidized mitochondrial nucleoids released by neutrophils drive type I interferon production in human lupus. J Exp Med 2016; 213: 697–713.
Schrader M, Yoon Y . Mitochondria and peroxisomes: are the 'big brother' and the 'little sister' closer than assumed? Bioessays 2007; 29: 1105–1114.
Bagattin A, Hugendubler L, Mueller E . Transcriptional coactivator PGC-1alpha promotes peroxisomal remodeling and biogenesis. Proc Natl Acad Sci USA 2010; 107: 20376–20381.
Berger J, Moller DE . The mechanisms of action of PPARs. Annu Rev Med 2002; 53: 409–435.
Costello JL, Castro IG, Hacker C, Schrader TA, Metz J, Zeuschner D et al. ACBD5 and VAPB mediate membrane associations between peroxisomes and the ER. J Cell Biol 2017; 216: 331–342.
Hua R, Cheng D, Coyaud E, Freeman S, Di Pietro E, Wang Y et al. VAPs and ACBD5 tether peroxisomes to the ER for peroxisome maintenance and lipid homeostasis. J Cell Biol 2017; 216: 367–377.
Stoica R, De Vos KJ, Paillusson S, Mueller S, Sancho RM, Lau KF et al. ER-mitochondria associations are regulated by the VAPB-PTPIP51 interaction and are disrupted by ALS/FTD-associated TDP-43. Nat Commun 2014; 5: 3996.
Giacomello M, Pellegrini L . The coming of age of the mitochondria-ER contact: a matter of thickness. Cell Death Differ 2016; 23: 1417–1427.
Sood A, Jeyaraju DV, Prudent J, Caron A, Lemieux P, McBride HM et al. A mitofusin-2-dependent inactivating cleavage of Opa1 links changes in mitochondria cristae and ER contacts in the postprandial liver. Proc Natl Acad Sci USA 2014; 111: 16017–16022.
Braverman NE, Moser AB . Functions of plasmalogen lipids in health and disease. Biochim Biophys Acta 2012; 1822: 1442–1452.
Herrera-Cruz MS, Simmen T . Of yeast, mice and men: MAMs come in two flavors. Biol Direct 2017; 12: 3.
Vance JE . Phospholipid synthesis in a membrane fraction associated with mitochondria. J Biol Chem 1990; 265: 7248–7256.
Vance JE . Newly made phosphatidylserine and phosphatidylethanolamine are preferentially translocated between rat liver mitochondria and endoplasmic reticulum. J Biol Chem 1991; 266: 89–97.
Vance JE . MAM (mitochondria-associated membranes) in mammalian cells: lipids and beyond. Biochim Biophys Acta 2014; 1841: 595–609.
Schrader M, Costello J, Godinho LF, Islinger M . Peroxisome-mitochondria interplay and disease. J Inherit Metab Dis 2015; 38: 681–702.
Pickrell AM, Youle RJ . The roles of PINK1, parkin, and mitochondrial fidelity in Parkinson's disease. Neuron 2015; 85: 257–273.
Area-Gomez E, Del Carmen Lara Castillo M, Tambini MD, Guardia-Laguarta C, de Groof AJ, Madra M et al. Upregulated function of mitochondria-associated ER membranes in Alzheimer disease. EMBO J 2012; 31: 4106–4123.
Area-Gomez E, Schon EA . Mitochondria-associated ER membranes and Alzheimer disease. Curr Opin Genet Dev 2016; 38: 90–96.
Vyas S, Zaganjor E, Haigis MC . Mitochondria and Cancer. Cell 2016; 166: 555–566.
Acknowledgements
We are grateful to Emma Clara Pellegrini for drawing the artwork shown in Figure 1.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Edited by G Melino
Rights and permissions
About this article
Cite this article
Schrader, M., Pellegrini, L. The making of a mammalian peroxisome, version 2.0: mitochondria get into the mix. Cell Death Differ 24, 1148–1152 (2017). https://doi.org/10.1038/cdd.2017.23
Received:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/cdd.2017.23
This article is cited by
-
Mitochondrial quality control in human health and disease
Military Medical Research (2024)
-
Organelle-targeted therapies: a comprehensive review on system design for enabling precision oncology
Signal Transduction and Targeted Therapy (2022)
-
The peroxisome: an update on mysteries 2.0
Histochemistry and Cell Biology (2018)