Abstract
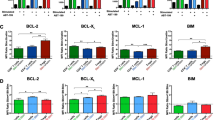
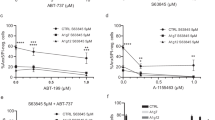
Survival of various immune cell populations has been proposed to preferentially rely on a particular anti-apoptotic BCL-2 family member, for example, naive T cells require BCL-2, while regulatory T cells require MCL-1. Here we examined the survival requirements of multiple immune cell subsets in vitro and in vivo, using both genetic and pharmacological approaches. Our findings support a model in which survival is determined by quantitative participation of multiple anti-apoptotic proteins rather than by a single anti-apoptotic protein. This model provides both an insight into how the sum of relative levels of anti-apoptotic proteins BCL-2, MCL-1 and A1 influence survival of T cells, B cells and dendritic cells, and a framework for ascertaining how these different immune cells can be optimally targeted in treatment of immunopathology, transplantation rejection or hematological cancers.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Czabotar PE, Lessene G, Strasser A, Adams JM . Control of apoptosis by the BCL-2 protein family: implications for physiology and therapy. Nat Rev Mol Cell Biol 2014; 15: 49–63.
Correia C, Lee SH, Meng XW, Vincelette ND, Knorr KL, Ding H et al. Emerging understanding of Bcl-2 biology: implications for neoplastic progression and treatment. Biochim Biophys Acta 2015; 1853: 1658–1671.
Moldoveanu T, Follis AV, Kriwacki RW, Green DR . Many players in BCL-2 family affairs. Trends Biochem Sci 2014; 39: 101–111.
Strasser A, Cory S, Adams JM . Deciphering the rules of programmed cell death to improve therapy of cancer and other diseases. EMBO J 2011; 30: 3667–3683.
Yuan S, Akey CW . Apoptosome structure, assembly, and procaspase activation. Structure 2013; 21: 501–515.
O'Neill KL, Huang K, Zhang J, Chen Y, Luo X . Inactivation of prosurvival Bcl-2 proteins activates Bax/Bak through the outer mitochondrial membrane. Genes Dev 2016; 30: 973–988.
Cartron PF, Gallenne T, Bougras G, Gautier F, Manero F, Vusio P et al. The first alpha helix of Bax plays a necessary role in its ligand-induced activation by the BH3-only proteins Bid and PUMA. Mol Cell 2004; 16: 807–818.
Kuwana T, Bouchier-Hayes L, Chipuk JE, Bonzon C, Sullivan BA, Green DR et al. BH3 domains of BH3-only proteins differentially regulate Bax-mediated mitochondrial membrane permeabilization both directly and indirectly. Mol Cell 2005; 17: 525–535.
Letai A, Bassik MC, Walensky LD, Sorcinelli MD, Weiler S, Korsmeyer SJ . Distinct BH3 domains either sensitize or activate mitochondrial apoptosis, serving as prototype cancer therapeutics. Cancer Cell 2002; 2: 183–192.
Chen L, Willis SN, Wei A, Smith BJ, Fletcher JI, Hinds MG et al. Differential targeting of prosurvival Bcl-2 proteins by their BH3-only ligands allows complementary apoptotic function. Mol Cell 2005; 17: 393–403.
Willis SN, Chen L, Dewson G, Wei A, Naik E, Fletcher JI et al. Proapoptotic Bak is sequestered by Mcl-1 and Bcl-xL, but not Bcl-2, until displaced by BH3-only proteins. Genes Dev 2005; 19: 1294–1305.
Krajewski S, Bodrug S, Gascoyne R, Berean K, Krajewska M, Reed JC . Immunohistochemical analysis of Mcl-1 and Bcl-2 proteins in normal and neoplastic lymph nodes. Am J Pathol 1994; 145: 515–525.
Krajewski S, Bodrug S, Krajewska M, Shabaik A, Gascoyne R, Berean K et al. Immunohistochemical analysis of Mcl-1 protein in human tissues. Differential regulation of Mcl-1 and Bcl-2 protein production suggests a unique role for Mcl-1 in control of programmed cell death in vivo. Am J Pathol 1995; 146: 1309–1319.
Vikstrom I, Carotta S, Luthje K, Peperzak V, Jost PJ, Glaser S et al. Mcl-1 is essential for germinal center formation and B cell memory. Science 2010; 330: 1095–1099.
Oltersdorf T, Elmore SW, Shoemaker AR, Armstrong RC, Augeri DJ, Belli BA et al. An inhibitor of Bcl-2 family proteins induces regression of solid tumours. Nature 2005; 435: 677–681.
Tse C, Shoemaker AR, Adickes J, Anderson MG, Chen J, Jin S et al. ABT-263: a potent and orally bioavailable Bcl-2 family inhibitor. Cancer Res 2008; 68: 3421–3428.
van Delft MF, Wei AH, Mason KD, Vandenberg CJ, Chen L, Czabotar PE et al. The BH3 mimetic ABT-737 targets selective Bcl-2 proteins and efficiently induces apoptosis via Bak/Bax if Mcl-1 is neutralized. Cancer Cell 2006; 10: 389–399.
Souers AJ, Leverson JD, Boghaert ER, Ackler SL, Catron ND, Chen J et al. ABT-199, a potent and selective BCL-2 inhibitor, achieves antitumor activity while sparing platelets. Nat Med 2013; 19: 202–208.
Roberts AW, Davids MS, Pagel JM, Kahl BS, Puvvada SD, Gerecitano JF et al. Targeting BCL2 with venetoclax in relapsed chronic lymphocytic leukemia. N Engl J Med 2016; 374: 311–322.
Stilgenbauer S, Eichhorst B, Schetelig J, Coutre S, Seymour JF, Munir T et al. Venetoclax in relapsed or refractory chronic lymphocytic leukaemia with 17p deletion: a multicentre, open-label, phase 2 study. Lancet Oncol 2016; 17: 768–778.
Roberts AW, Seymour JF, Brown JR, Wierda WG, Kipps TJ, Khaw SL et al. Substantial susceptibility of chronic lymphocytic leukemia to BCL2 inhibition: results of a phase I study of navitoclax in patients with relapsed or refractory disease. J Clin Oncol 2012; 30: 488–496.
Mason KD, Carpinelli MR, Fletcher JI, Collinge JE, Hilton AA, Ellis S et al. Programmed anuclear cell death delimits platelet life span. Cell 2007; 128: 1173–1186.
Zhang H, Nimmer PM, Tahir SK, Chen J, Fryer RM, Hahn KR et al. Bcl-2 family proteins are essential for platelet survival. Cell Death Differ 2007; 14: 943–951.
Bouillet P, Cory S, Zhang LC, Strasser A, Adams JM . Degenerative disorders caused by Bcl-2 deficiency prevented by loss of its BH3-only antagonist Bim. Dev Cell 2001; 1: 645–653.
Nakayama K, Nakayama K, Negishi I, Kuida K, Sawa H, Loh DY . Targeted disruption of Bcl-2 alpha beta in mice: occurrence of gray hair, polycystic kidney disease, and lymphocytopenia. Proc Natl Acad Sci USA 1994; 91: 3700–3704.
Carrington EM, Vikstrom IB, Light A, Sutherland RM, Londrigan SL, Mason KD et al. BH3 mimetics antagonizing restricted prosurvival Bcl-2 proteins represent another class of selective immune modulatory drugs. Proc Natl Acad Sci USA 2010; 107: 10967–10971.
Carrington EM, Zhang JG, Sutherland RM, Vikstrom IB, Brady JL, Soo P et al. Prosurvival Bcl-2 family members reveal a distinct apoptotic identity between conventional and plasmacytoid dendritic cells. Proc Natl Acad Sci USA 2015; 112: 4044–4049.
Peperzak V, Vikstrom I, Walker J, Glaser SP, LePage M, Coquery CM et al. Mcl-1 is essential for the survival of plasma cells. Nat Immunol 2013; 14: 290–297.
Sathe P, Delconte RB, Souza-Fonseca-Guimaraes F, Seillet C, Chopin M, Vandenberg CJ et al. Innate immunodeficiency following genetic ablation of Mcl1 in natural killer cells. Nat Commun 2014; 5: 4539.
Pierson W, Cauwe B, Policheni A, Schlenner SM, Franckaert D, Berges J et al. Antiapoptotic Mcl-1 is critical for the survival and niche-filling capacity of Foxp3(+) regulatory T cells. Nat Immunol 2013; 14: 959–965.
Kotschy A, Szlavik Z, Murray J, Davidson J, Maragno AL, Le Toumelin-Braizat G et al. The MCL1 inhibitor S63845 is tolerable and effective in diverse cancer models. Nature 2016; 538: 477–482.
Schenk RL, Tuzlak S, Carrington EM, Zhan Y, Heinzel S, Teh CE et al. Characterisation of mice lacking all functional isoforms of the pro-survival BCL-2 family member A1 reveals minor defects in the haematopoietic compartment. Cell Death Differ 2017; 24: 534–545.
Tuzlak S, Schenk RL, Vasanthakumar A, Preston SP, Haschka MD, Zotos D et al. The BCL-2 pro-survival protein A1 is dispensable for T cell homeostasis on viral infection. Cell Death Differ 2017; 24: 523–533.
Vikstrom IB, Slomp A, Carrington EM, Moesbergen LM, Chang C, Kelly GL et al. MCL-1 is required throughout B-cell development and its loss sensitizes specific B-cell subsets to inhibition of BCL-2 or BCL-XL. Cell Death Dis 2016; 7: e2345.
Khaw SL, Merino D, Anderson MA, Glaser SP, Bouillet P, Roberts AW et al. Both leukaemic and normal peripheral B lymphoid cells are highly sensitive to the selective pharmacological inhibition of prosurvival Bcl-2 with ABT-199. Leukemia 2014; 28: 1207–1215.
Merino D, Khaw SL, Glaser SP, Anderson DJ, Belmont LD, Wong C et al. Bcl-2, Bcl-x(L), and Bcl-w are not equivalent targets of ABT-737 and navitoclax (ABT-263) in lymphoid and leukemic cells. Blood 2012; 119: 5807–5816.
Yang T, Kozopas KM, Craig RW . The intracellular distribution and pattern of expression of Mcl-1 overlap with, but are not identical to, those of Bcl-2. J Cell Biol 1995; 128: 1173–1184.
Adams KW, Cooper GM . Rapid turnover of mcl-1 couples translation to cell survival and apoptosis. J Biol Chem 2007; 282: 6192–6200.
Akgul C, Moulding DA, White MR, Edwards SW . In vivo localisation and stability of human Mcl-1 using green fluorescent protein (GFP) fusion proteins. FEBS Lett 2000; 478: 72–76.
Herold MJ, Zeitz J, Pelzer C, Kraus C, Peters A, Wohlleben G et al. The stability and anti-apoptotic function of A1 are controlled by its C terminus. J Biol Chem 2006; 281: 13663–13671.
Kucharczak JF, Simmons MJ, Duckett CS, Gelinas C . Constitutive proteasome-mediated turnover of Bfl-1/A1 and its processing in response to TNF receptor activation in FL5.12 pro-B cells convert it into a prodeath factor. Cell Death Differ 2005; 12: 1225–1239.
Chao JR, Wang JM, Lee SF, Peng HW, Lin YH, Chou CH et al. mcl-1 is an immediate-early gene activated by the granulocyte-macrophage colony-stimulating factor (GM-CSF) signaling pathway and is one component of the GM-CSF viability response. Mol Cell Biol 1998; 18: 4883–4898.
Lin EY, Orlofsky A, Berger MS, Prystowsky MB . Characterization of A1, a novel hemopoietic-specific early-response gene with sequence similarity to bcl-2. J Immunol 1993; 151: 1979–1988.
He CH, Waxman AB, Lee CG, Link H, Rabach ME, Ma B et al. Bcl-2-related protein A1 is an endogenous and cytokine-stimulated mediator of cytoprotection in hyperoxic acute lung injury. J Clin Invest 2005; 115: 1039–1048.
Olsson Akefeldt S, Maisse C, Belot A, Mazzorana M, Salvatore G, Bissay N et al. Chemoresistance of human monocyte-derived dendritic cells is regulated by IL-17A. PloS ONE 2013; 8: e56865.
Le Gouill S, Podar K, Amiot M, Hideshima T, Chauhan D, Ishitsuka K et al. VEGF induces Mcl-1 up-regulation and protects multiple myeloma cells against apoptosis. Blood 2004; 104: 2886–2892.
Zhang J, D'Ercole AJ . Expression of Mcl-1 in cerebellar granule neurons is regulated by IGF-I in a developmentally specific fashion. Brain Res Dev Brain Res 2004; 152: 255–263.
Touzeau C, Dousset C, Le Gouill S, Sampath D, Leverson JD, Souers AJ et al. The Bcl-2 specific BH3 mimetic ABT-199: a promising targeted therapy for t(11;14) multiple myeloma. Leukemia 2014; 28: 210–212.
Rinkenberger JL, Horning S, Klocke B, Roth K, Korsmeyer SJ . Mcl-1 deficiency results in peri-implantation embryonic lethality. Genes Dev 2000; 14: 23–27.
Ogilvy S, Metcalf D, Print CG, Bath ML, Harris AW, Adams JM . Constitutive Bcl-2 expression throughout the hematopoietic compartment affects multiple lineages and enhances progenitor cell survival. Proc Natl Acad Sci USA 1999; 96: 14943–14948.
Campbell KJ, Bath ML, Turner ML, Vandenberg CJ, Bouillet P, Metcalf D et al. Elevated Mcl-1 perturbs lymphopoiesis, promotes transformation of hematopoietic stem/progenitor cells, and enhances drug resistance. Blood 2010; 116: 3197–3207.
Vandenberg CJ, Cory S . ABT-199, a new Bcl-2-specific BH3 mimetic, has in vivo efficacy against aggressive Myc-driven mouse lymphomas without provoking thrombocytopenia. Blood 2013; 121: 2285–2288.
Koenen P, Heinzel S, Carrington EM, Happo L, Alexander WS, Zhang JG et al. Mutually exclusive regulation of T cell survival by IL-7R and antigen receptor-induced signals. Nat Commun 2013; 4: 1735.
Lang MJ, Brennan MS, O'Reilly LA, Ottina E, Czabotar PE, Whitlock E et al. Characterisation of a novel A1-specific monoclonal antibody. Cell Death Dis 2014; 5: e1553.
Vremec D, Pooley J, Hochrein H, Wu L, Shortman K . CD4 and CD8 expression by dendritic cell subtypes in mouse thymus and spleen. J Immunol 2000; 164: 2978–2986.
Acknowledgements
We thank M Dayton, M Iliopoulos, T Mason, H Johnson and M Hancock for technical assistance. This work was supported by Rebecca L. Cooper Foundation, National Health and Medical Research Council of Australia (NHMRC) grants and fellowships #1037321, #1043414, #1080321 and #1105209 to AML, #461221 and #1042629 to PB, #1020363 and #1016701 to AS, #1054925 and #1060675 to DT, #1016647 to JGZ, #1049720 to MH, the Leukemia and Lymphoma Society (LLS) Specialized Center of Research grant (#7001-13 to AS), Cancer Council Victoria (CCV) Grant-in-aid (#1102104 to AS), Austrian Academy of Science (ÖAW) Doc-fellowship (to MH), Leukemia Foundation National Research Program PhD Scholarship (to RS), NHMRC Independent Research Institutes Infrastructure Support Scheme grant (361646) and Victorian State Government Operational Infrastructure Support grant. We acknowledge the Wurundjeri people of the Kulin nation as the traditional owners and custodians of the land on which most of the work was performed.
Author contributions
Designed research: EMC, AML, IBV, DMT, JGZ and YZ. Performed research: EMC, JLB, JGZ and RMS. Contributed vital new reagents or analytical tools: NSA, RLS, MJH, RBD, NDH, DS, DCSH, SC, PB and AS. Collected, analyzed and interpreted data: EMC, JLB, JGZ, RMS and AML. Wrote the manuscript: EMC, AML, AS, DMT, SC and YZ.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
All relevant authors are employees of The Walter and Eliza Hall Institute. This institute receives milestone payments for the development of BH3 mimetic drugs for therapy from Genentech and AbbVie.
Additional information
Edited by H Steller
Supplementary Information accompanies this paper on Cell Death and Differentiation website
Supplementary information
Rights and permissions
About this article
Cite this article
Carrington, E., Zhan, Y., Brady, J. et al. Anti-apoptotic proteins BCL-2, MCL-1 and A1 summate collectively to maintain survival of immune cell populations both in vitro and in vivo. Cell Death Differ 24, 878–888 (2017). https://doi.org/10.1038/cdd.2017.30
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/cdd.2017.30
This article is cited by
-
DrugBank mining with machine learning reveals novel candidates for BCL-2 inhibition
Scientific Reports (2026)
-
Dual Bcl-2/Bcl-xl inhibition via AZD0466 combines with immune checkpoint blockade to enhance anti-tumour activity
Cell Death & Disease (2026)
-
A new era in cancer therapy: targeting the Proteasome-Bcl-2 axis
Journal of Experimental & Clinical Cancer Research (2025)
-
Advancing CAR-based cell therapies for solid tumours: challenges, therapeutic strategies, and perspectives
Molecular Cancer (2025)
-
Rational engineering of nanoplatforms with hierarchical targeting hepatic tumor and subcellular mitochondria for enhanced image-guided photodynamic therapy
Journal of Nanobiotechnology (2025)