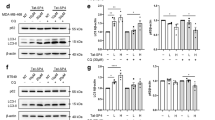
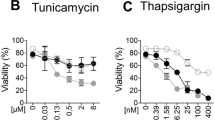
Abstract
Programmed cell death, which is required for the development and homeostasis of metazoans, includes mechanisms such as apoptosis, autophagic cell death, and necrotic (or type III) death. Members of the Bcl2 family regulate apoptosis, among which Bax and Bak act as a mitochondrial gateway. Although embryonic fibroblasts from Bax/Bak double-knockout (DKO) mice are resistant to apoptosis, we previously demonstrated that these cells die through an autophagy-dependent mechanism in response to various types of cellular stressors. To determine the physiological role of autophagy-dependent cell death, we generated Atg5/Bax/Bak triple-knockout (TKO) mice, in which autophagy is greatly suppressed compared with DKO mice. Embryonic fibroblasts and thymocytes from TKO mice underwent autophagy much less frequently, and their viability was much higher than DKO cells in the presence of certain cellular stressors, providing genetic evidence that DKO cells undergo Atg5-dependent death. Compared with wild-type embryos, the loss of interdigital webs was significantly delayed in DKO embryos and was even further delayed in TKO embryos. Brain malformation is a distinct feature observed in DKO embryos on the 129 genetic background, but not in those on a B6 background, whereas such malformations appeared in TKO embryos even on a B6 background. Taken together, our data suggest that Atg5-dependent cell death contributes to the embryonic development of DKO mice, implying that autophagy compensates for the deficiency in apoptosis.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Clarke PG . Developmental cell death: morphological diversity and multiple mechanisms. Anat Embryol 1990; 181: 195–213.
Tsujimoto Y . Cell death regulation by the Bcl-2 protein family in the mitochondria. J Cell Physiol 2003; 195: 158–167.
Baehrecke EH . How death shapes life during development. Nat Rev Mol Cell Biol 2002; 3: 779–787.
Das G, Shravage BV, Baehrecke EH . Regulation and function of autophagy during cell survival and cell death. Cold Spring Harb Perspect Biol 2012; 4: a008813.
Xie Z, Klionsky DJ . Autophagosome formation: core machinery and adaptations. Nat Cell Biol 2007; 9: 1102–1109.
Mizushima N, Yoshimori T, Ohsumi Y . The role of Atg proteins in autophagosome formation. Annu Rev Cell Dev Biol 2011; 27: 107–132.
Nishida Y, Arakawa S, Fujitani K, Yamaguchi H, Mizuta T, Kanaseki T et al. Discovery of Atg5/Atg7-independent alternative macroautophagy. Nature 2009; 461: 654–658.
Levine B, Yuan J . Autophagy in cell death: an innocent convict? J Clin Invest 2005; 115: 2679–2688.
Shen HM, Codogno P . Autophagic cell death: Loch Ness monster or endangered species? Autophagy 2011; 7: 457–465.
Denton D, Nicolson S, Kumar S . Cell death by autophagy: facts and apparent artifacts. Cell Death Differ 2012; 19: 87–95.
Tracy K, Baehrecke EH . The role of autophagy in Drosophila metamorphosis. Curr Top Dev Biol 2013; 103: 101–125.
Shimizu S, Kanaseki T, Mizushima N, Mizuta T, Arakawa-Kobayashi S, Thompson CB et al. Role of Bcl-2 family proteins in a non-apoptotic programmed cell death dependent on autophagy genes. Nat Cell Biol 2004; 6: 1221–1228.
Eisenberg-Lerner A, Bialik S, Simon HU, Kimchi A . Life and death partners: apoptosis, autophagy and the cross-talk between them. Cell Death Differ 2009; 16: 966–975.
Kuma A, Hatano M, Matsui M, Yamamoto A, Nakaya H, Yoshimori T et al. The role of autophagy during the early neonatal starvation period. Nature 2004; 432: 1032–1036.
Degterev A, Huang Z, Boyce M, Li Y, Jagtap P, Mizushima N et al. Chemical inhibitor of nonapoptotic cell death with therapeutic potential for ischemic brain injury. Nat Chem Biol 2005; 1: 112–119.
Liu Y, Shoji-Kawata S, Sumpter RM Jr, Wei Y, Ginet V, Zhang L et al. Autosis is a Na+, K+-ATPase-regulated form of cell death triggered by autophagy-inducing peptides, starvation, and hypoxia-ischemia. Proc Natl Acad Sci USA 2013; 110: 20364–20371.
Nakagawa T, Shimizu S, Watanabe T, Yamaguchi O, Otsu K, Yamagata H et al. Cyclophilin D-dependent mitochondrial permeability transition regulates some necrotic but not apoptotic cell death. Nature 2005; 434: 652–658.
Zong WX, Ditsworth D, Bauer DE, Wang Z-Q, Thompson CB . Alkylating DNA damage stimulates a regulated form of necrotic cell death. Genes Dev 2004; 18: 1272–1282.
Boya P, Kroemer G . Lysosomal membrane permeabilization in cell death. Oncogene 2008; 27: 6434–6451.
Kaufmann SH . Induction of endonucleolytic DNA cleavage in human acute myelogenous leukemia cells by etoposide, camptothecin, and other cytotoxic anticancer drugs: a cautionary note. Cancer Res 1989; 49: 5870–5878.
Rathmell JC, Lindsten T, Zong W-X, Cinalli RM, Thompson CB . Deficiency in Bak and Bax perturbs thymic selection and lymphoid homeostasis. Nat Immunol 2002; 3: 932–939.
Houde C, Banks KG, Coulombe N, Rasper D, Grimm E, Roy S et al. Caspase-7 expanded function and intrinsic expression level underlies strain-specific brain phenotype of caspase-3-null mice. J Neurosci 2004; 24: 9977–9984.
Lindsten T, Ross AJ, King A, Zong WX, Rathmell JC, Shiels HA et al. The combined functions of proapoptotic Bcl-2 family members Bak and Bax are essential for normal development of multiple tissues. Mol Cell 2000; 6: 1389–1399.
Miyanishi M, Tada K, Koike M, Uchiyama Y, Kitamura T, Nagata S . Identification of Tim4 as a phosphatidylserine receptor. Nature 2007; 450: 435–439.
Yamaguchi Y, Shinotsuka N, Nonomura K, Takemoto K, Kuida K, Yosida H et al. Live imaging of apoptosis in a novel transgenic mouse highlights its role in neural tube closure. J Cell Biol 2011; 195: 1047–1060.
Yoshida H, Kong YY, Yoshida R, Elia AJ, Hakem A, Hakem R et al. Apaf1 is required for mitochondrial pathways of apoptosis and brain development. Cell 1998; 94: 739–750.
Harris MJ, Juriloff DM . Mouse mutants with neural tube closure defects and their role in understanding human neural tube defects. Birth Defects Res A Clin Mol Teratol 2007; 79: 187–210.
Nagasaka A, Kawane K, Yoshida H, Nagata S . Apaf-1-independent programmed cell death in mouse development. Cell Death Differ 2010; 17: 931–941.
Newton K, Sun X, Dixit VM . Kinase RIP3 is dispensable for normal NF-κBs, signaling by the B-cell and T-cell receptors, tumor necrosis factor receptor 1, and toll-like receptors 2 and 4. Mol Cell Biol 2004; 24: 1464–1469.
Wu J, Huang Z, Ren J, Zhang Z, He P, Li Y et al. Mlkl knockout mice demonstrate the indispensable role of Mlkl in necroptosis. Cell Res 2013; 23: 994–1006.
Kabeya Y, Mizushima N, Ueno T, Yamamoto A, Kirisako T, Noda T et al. LC3, a mammalian homologue of yeast Apg8p, is localized in autophagosome membranes after processing. EMBO J 2000; 19: 5720–5728.
Acknowledgements
We are grateful to Dr N. Mizushima for providing us with Atg5+/− mice. This study was supported in part by Grant-in-Aid for Grant-in-Aid for Scientific Research (S) (22229002), Grant-in-Aid for Scientific Research (A) (17H01533), Grant-in-Aid for challenging Exploratory Research (16K15230), Grant-in-Aid for Scientific Research on Innovative Areas (15H01554, 26110001, 26110005), Grant-in-Aid for Encouragement of Young Scientists (B) (15K19004) from the MEXT of Japan, by the Project for Cancer Research and Therapeutic Evolution (P-CREATE) and by the Project for Psychiatric and Neurological Disorders from the Japan Agency for Medical Research and development, AMED. This study was also supported by the Joint Usage/Research Program of Medical Research Institute, Tokyo Medical and Dental University, and by a grant from the Secom Science and Technology Foundation and the Takeda Science Foundation.
Author contributions
SA performed EM analyses. MT, TY, HS, YN, YM, IY, and SS performed other experiments and analyzed the results. YT and SS designed and conducted the research, and wrote the paper.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Edited by A Villunger
Supplementary Information accompanies this paper on Cell Death and Differentiation website
Supplementary information
Rights and permissions
About this article
Cite this article
Arakawa, S., Tsujioka, M., Yoshida, T. et al. Role of Atg5-dependent cell death in the embryonic development of Bax/Bak double-knockout mice. Cell Death Differ 24, 1598–1608 (2017). https://doi.org/10.1038/cdd.2017.84
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/cdd.2017.84
This article is cited by
-
Ginsenoside Rh2 regulates cardiomyocyte autophagy-dependent apoptosis through the PI3K-Akt-mTOR signaling pathway to attenuate doxorubicin-induced cardiotoxicity
Applied Biological Chemistry (2025)
-
The BCL2 family: from apoptosis mechanisms to new advances in targeted therapy
Signal Transduction and Targeted Therapy (2025)
-
Sigma-1 Receptor Ligands for CNS Cancer Treatment
CNS Drugs (2025)
-
Comprehensive analysis of regulated cell death pathways: intrinsic disorder, protein–protein interactions, and cross-pathway communication
Apoptosis (2025)
-
Autophagy–lysosomal-associated neuronal death in neurodegenerative disease
Acta Neuropathologica (2024)