Abstract
The mammalian growth factor erv1-like (GFER) gene encodes a sulfhydryl oxidase enzyme, named Augmenter of Liver Regeneration (ALR). Recently it has been demonstrated that ALR supports cell proliferation acting as an anti-apoptotic factor. This effect is determined by ALR ability to support the anti-apoptotic gene expression and to preserve cellular normoxic conditions. We recently demonstrated that the addition of recombinant ALR (rALR) in the culture medium of H2O2-treated neuroblastoma cells reduces the lethal effects induced by the hydrogen peroxide. Similar data have been reported in the regenerating liver tissue from partially hepatectomized rats treated with rALR. The purpose of the present study was to evaluate the effect of the GFER inhibition, via the degradation of the complementary mRNA by the specific siRNA, on the behaviour of the apoptosis (apoptotic gene and caspase expression and apoptotic cell number) and of the oxidative stress-induced parameters (reactive oxygen species (ROS), clusterin expression and mitochondrial integrity) in T98G glioma cells. The results revealed a reduction of (i) ALR, (ii) clusterin and (iii) bcl-2 and an increase of (iv) caspase-9, activated caspase-3, ROS, apoptotic cell number and mitochondrial degeneration. These data confirm the anti-apoptotic role of ALR and its anti-oxidative properties, and shed some light on the molecular pathways through which ALR modulates its biological effects.
Similar content being viewed by others
Main
Gliomas represent the most frequently diagnosed tumours of the central nervous system. With a highly invasive phenotype, gliomas diffusely infiltrate into various regions of the brain,1, 2 rendering their surgical resection impossible; therefore, the prognosis for patients with gliomas is poor even in response to multidisciplinary treatment.3
Augmenter of Liver Regeneration (ALR), a sulfhydryl oxidase enzyme4 encoded by the growth factor erv1-like (GFER) gene, is present in all mammalian cells5, 6, 7 and, compared with tissues from healthy subjects, is significantly overexpressed in tissues of patients affected by cell-proliferating diseases, such as hepatocellular carcinoma (HCC), chronic hepatitis (CH), cholangiocellular carcinoma8 or in regenerating liver after partial hepatectomy in rats.9, 10 We recently demonstrated that ALR protects human-derived neuroblastoma cells from H2O2-induced apoptosis, reducing mitochondrial swelling, cell death and apoptotic cell number, inhibiting cytochrome-c mitochondrial release and upregulating the bcl-2/bax gene-transcription protein ratio. Similar data are reported in the literature in different experimental models, in vivo and in vitro.11, 12, 13, 14, 15
Various reports reveal that a defective or inefficient apoptosis is an acquired hallmark of cancer cells,16 which renders any pharmacological treatment almost ineffective.17 Apoptosis is characterized by two different molecular pathways: (i) the extrinsic pathway, which is triggered by the presence of transmembrane death receptors (Fas, TNF receptor and TRAIL receptors) and their respective ligands on cellular membranes and (ii) the intrinsic pathway, known as mitochondrial apoptosis, which requires the disruption of the mitochondrial outer membrane and leads to the apoptotic caspase cascade.18 Mitochondrial apoptosis is regulated by the anti-apoptotic bcl-2 family of proteins, which prevents mitochondrial swelling.
In the present study, in human-derived glioma cells, we investigated the effect of GFER silencing, by specific siRNA, on apoptotic gene expression, caspase-9 and activated caspase-3 expression, apoptotic cell number, reactive oxygen species (ROS) levels, clusterin expression and mitochondrial morphology.
Results
H2O2-induced oxidative stress
Figure 1 reports the behaviour of H2O2-induced ROS in glioma cells maintained in cell culture and the effect of the increasing doses of recombinant ALR (rALR) to the culture medium. We have previously and broadly described the effects of ALR both on apoptosis and on the endocellular redox state altered by H2O2 addition in cell culture of neuroblastoma cells.12 In our present experimental conditions, a five- to six-fold increase, statistically significant (P=3.7 × 10−5), of ROS levels was registered in H2O2-treated cells compared with H2O2-untreated cells. The presence of increasing doses of rALR in the culture medium counteracted the effect induced by H2O2.
Effect of GFER silencing
ALR expression
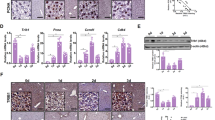
Figure 2 reports ALR expression in siRNA/ALR-treated cells and control cells evaluated both using western blot analysis (Figure 2a) and confocal microscopy (Figure 2b). The western blot analysis revealed the presence of two forms of the protein, one of 21 and one of 23 kDa,10 markedly reduced in siRNA/ALR-treated cells compared with control cells (Figure 2a, upper-right). The normalization of ALR expression, with respect to β-actin level, revealed a statistically significant (P=6.7 × 10−5) reduction of ALR in siRNA/ALR-treated cells compared with control cells. The confocal microscopy immunodetection revealed a considerable presence of ALR in control cells, which resulted significantly reduced in siRNA/ALR-treated cells (Figure 2b). For each determination, the nuclear identification, evidenced by TO-PRO-3 (Invitrogen srl, Milan, Italy), the specific ALR immunodetection, revealed by Alexa Fluor 488 (Invitrogen srl), and the ‘merge’ are reported.
Clusterin expression
Figure 3 reports the clusterin expression in siRNA/ALR-treated and control glioma cells evaluated using western blot analysis (Figure 3a) and confocal microscopy (Figure 3b). A marked reduction of clusterin in siRNA/ALR-treated cells, compared with control cells, was evidenced (Figure 3a, upper-right). The normalization of clusterin expression, compared with β-actin level, revealed a statistically significant (P=5.4 × 10−3) reduction of the protein. Clusterin immunodetection using confocal microscopy revealed a considerable amount of this factor in the cytosol of control glioma cells, which dramatically reduced in siRNA/ALR-treated cells (Figure 3b). Nuclear identification, evidenced by TO-PRO-3, and the specific clusterin immunodetection, evidenced by Alexa Fluor 488, are reported.
Effect of siRNA/ALR treatment on clusterin expression. Clusterin expression in siRNA/ALR-treated and control human T98G glioma cells, determined using western blot analysis (a, upper right) and confocal microscopy (b, green colour), are reported. The data shown are means±S.D. from three independent experiments
ROS levels
Figure 4 reports the level of ROS determined by the GFER siRNA treatment and the protective effect of rALR when it was introduced into the culture medium. A statistically significant (P=1.1 × 10−4) increase of >4-fold of the ROS was present in siRNA/ALR-treated cells compared with control cells. The presence of increasing doses of rALR (1–100 ng/ml) in the culture medium counteracted the siRNA/ALR-induced oxidation in a dose-dependent way.
Effect of siRNA/ALR treatment on ROS levels expressed by glioma cells. siRNA/ALR-treated cells were incubated with different concentrations of rALR (1–100 ng/ml), subsequently loaded with DCF-DA and fluorescence intensity measured as described in Materials and Methods. Means±S.D. of six triplicate independent experiments are shown
Apoptotic gene expression
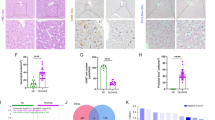
Figure 5 reports the effect of GFER siRNA treatment on the expression of apoptotic genes, bcl-2 and bax (Figure 5a), caspase-9 (Figure 5b) and activated caspase-3 (Figure 5c) in siRNA/ALR-treated and control glioma cells. The western blot analysis of bcl-2 and bax (Figure 5a) showed a significant reduction of bcl-2 expression in siRNA/ALR-treated cells, compared with control cells, without a notable variation of bax between the two experimental conditions. The densitometric analysis of the bcl-2 signals, normalized to β-actin expression, demonstrated that the bcl-2 expression was significantly reduced in siRNA/ALR-treated cells (P=4.1 × 10−5). Figure 5b reports caspase-9 expression revealing its increase in siRNA/ALR-treated cells, compared with control cells. The densitometric analysis, normalized to β-actin, revealed this variation statistically significant (P=1.4 × 10–3). Figure 5c reports the expression of activated caspase-3. An increase was detected in siRNA/ALR-treated cells, compared with control cells, and the densitometric analysis of the protein-related signals, normalized with that of the β-actin, revealed the increase statistically significant (P=1.3 × 10−5).
Effect of siRNA/ALR treatment on apoptotic gene expression. bcl-2 and bax (a), caspase-9 (b) and activated caspase-3 (c) expression in siRNA/ALR-treated and control human T98G glioma cells, determined using western blot analysis, are reported. The data shown are means±S.D. from five independent experiments
Cytofluorimetric analysis
Figure 6 reports the cytofluorimetric analysis of siRNA/ALR-treated and control glioma cells. In the upper part of the figure (Figure 6a), the biparametric histograms LOG FL1 versus LOG FL4 are reported, distinguishing four cell populations: the viable cells (low FITC and low 7-AAD), the secondary necrotic cells (high FITC and high 7-AAD), the early apoptotic cells (high FITC and low 7-AAD) and a possible fourth cell population corresponding to the damaged viable cells (low FITC and high 7-AAD). An increase in the percentage of early apoptotic cells was observed in the culture of siRNA/ALR-treated glioma cells and the comparison of the data from three different experiments (Figure 6b) revealed a statistically different (P=1.9 × 10−8) percentage of Annexin V+ cells in the culture of siRNA/ALR-treated cells (40.72±3.5) compared with the percentage of Annexin V+ cells detected in the culture of control cells (5.63±1.55). No necrotic or damaged cells were detected in any evaluation.
Effect of siRNA/ALR treatment on cytofluorimetric analysis of apoptotic glioma cells. In the upper part of the figure (a), the biparametric histogram of LOG FL1 versus LOG FL4, in the siRNA/ALR-treated and control cells, is reported. A lower percentage of Annexin V+ cells in the culture of control cells (5.63±1.55) compared with the percentage of Annexin V+ cells in the culture of siRNA/ALR-treated cells (40.72±3.5) is observed (b). The data shown are the mean±S.D. from three independent experiments
Electron microscopy (EM) analysis
Figures 7 and 8 report the analysis, performed via EM, of control glioma (Figure 7) and siRNA/ALR-treated cells (Figure 8). The control cells appear elongated with peripheral cytoplasmic expansion and the nuclei contain finely aggregated chromatin (down-left inset) and voluminous nucleoli (arrows). The cytoplasm contains numerous mitochondria of normal appearance (up-right inset). Instead, the siRNA/ALR-treated cells present a more abundant cytoplasm with degenerated organelles. The nuclei are extremely irregular and the mitochondria appear swollen or fragmented (down-right inset). The cells present electron-dense granules resembling lysosome structures (up-left inset). Vacuolar dilatations of the endoplasmic reticulum are present (arrows).
Effect of siRNA/ALR treatment on glioma cell morphology determined by EM. The siRNA/ALR-treated glioma cells present degenerated organules in the cytoplasm ( × 4400), nuclei extremely irregular, mitochondria swollen or fragmented (right-down insert; × 56 000) and electron-dense granules resembling lysosome (up-left insert; × 11 000). Vacuolar dilatations of the endoplasmic reticulum are present (arrows)
Discussion
More than 10 years ago, our group identified a new protein, the ALR,5, 19 initially known as HSS,20 which increases hepatocyte proliferation when administered to liver cells already primed to proliferate;21 the exact role of ALR in the cellular metabolism is still not well defined. Only recently, some of the metabolic functions of ALR have been revealed by in vivo and in vitro10, 11, 12, 13, 14, 15 experiments. In a recent study, in partially hepatectomized rats,10 we demonstrated a rapid increase of ALR in the regenerating liver in the first 6–12 h after the surgery, which favours the anti-apoptotic gene expression, a molecular event, at this time of the regenerative process, crucial for the sustention of liver-mass recovery after the surgery.9, 22 In the same study, we identified two ALR protein isoforms, with molecular weights of 21 and 23 kDa, respectively:10 the 21-kDa form significantly increased after partial hepatectomy (PH), supporting the notion that this could be the ALR protein form that actively participates in the regenerative process after PH, whereas the 23-kDa form remains unchanged. This latter molecular form of ALR has been predominantly recognized in the mitochondrial IMS23 where it is involved (i) through its FAD molecule24, 25 in the electron pathway, with cytochrome-c25 and the Mia4026 as physiological partners and (ii) in the maturation of cytosolic Fe/S proteins.27 Moreover, for the first time in vivo, we demonstrated the anti-oxidative capacity of ALR, a biological effect achieved by the induction of clusterin, a secreted chaperone capable of maintaining the physiological cellular redox state.28
In our experiments, as other investigators,4, 10, 12, 14, 15, 29 we used a small 15-kDa recombinant form of ALR that, mainly reported as an extracellular cytokine,12, 26 represents the smallest yet functional protein capable of participating in the intracellular redox-dependent signalling pathways.12 Indeed, the small 15-kDa ALR form, similarly to other ALR homologous proteins identified in all organisms from viruses to mammals,6 maintains a well conserved amino-acid domain, CxxC, at the C-terminus part of the molecule, responsible for its sulfhydryl oxidase enzymatic activity.4, 24, 25, 26, 27 All these proteins have different molecular weight and cytoplasmic localization, but probably all support similar biological functions as has been reported for some.4, 23, 24, 25, 26, 27 It is noteworthy to underscore the fact that the most important signal structures for the interactions and subcellular distribution of ALR are located at the C-terminal domain of the protein and not at the N-terminal domain.23, 24, 25, 26
An additional finding that can help for the identification of the role of ALR in the cellular metabolism comes from in vitro studies. Analyzing the H2O2-induced apoptotic process in human-derived neuroblastoma cells, we demonstrated that the addition of rALR in the culture medium normalizes LPO, protein carbonylation and mitochondrial membrane permeability altered by the presence of H2O2.12 All these parameters are typical of the mitochondrial apoptosis.
Furthermore, we demonstrated that ALR supports the OXPHOS and ATP production in mitochondria isolated from intact liver of rat intraperitoneally treated with rALR; these effects are achieved by (i) the induction of mtTFA, a mitochondrial DNA regulatory factor, and (ii) the upregulation of mitochondrial gene expression.30
In the present study, we tested the effect of ALR gene-silencing (GFER) on the cell morphology, mitochondrial apoptosis and cellular redox state of human-derived glioma cells, T98G, maintained in culture. The data obtained demonstrated a significant reduction of ALR protein in siRNA/ALR-treated cells (Figures 2a and b) and, as a consequence, (i) a decrease of clusterin expression (Figures 3a and b), an increase of (ii) ROS levels (Figure 4) and (iii) the apoptotic process, evaluated by bcl-2, bax (Figure 5a), caspase-9 (Figure 5b), activated caspase-3 protein expression (Figure 5c) and the number of the apoptotic cells (Figure 6). The induction of these biological events sufficiently justifies the cellular morphological alterations evidenced in the GFER-silenced glioma cells (Figure 8) compared with control cells (Figure 7). In addition, we demonstrated that the presence of increasing concentrations of rALR into the culture medium of siRNA/ALR-treated cells abolished, almost totally, the increased level of ROS induced by the siRNA/ALR treatment (Figure 4). As mentioned before, we already reported the beneficial effects of ALR on the cell redox state and on apoptosis.10
To efficiently collocate these data within the cell metabolism, some important considerations are necessary. The devastating effect on cell metabolism of ROS increase, associated to the induction of apoptosis, has been unanimously reported under many experimental conditions.17, 31 It is known that the ‘oxidative stress’ can cause DNA, protein, and/or lipid damage, leading to changes in chromosome stability, genetic mutation, and/or modulation of cell growth that may result in cancer.32 Recent data underlined the strong relationship between increased ROS levels and neoplastic transformation, attributing them the role of critical signalling molecules in cancer cells as well as in metastatic cells.33 Indeed, such a state has been shown to regulate both genetic and epigenetic cascades, and mutation studies have suggested that chronic oxidative stress, particularly from chronic inflammation, is associated with carcinogenesis.34 For example, ulcerative colitis has long been linked with a high incidence of colorectal cancer and chronic gastritis due to, for instance, the infection with Helicobacter pylori, with the gastric cancer,34 as well as HCC in patients affected by CH. Most of these pathological conditions are associated with high ALR serum levels.8
Various reports reveal a defective or inefficient apoptosis of cancer cells,16, 35, 36 which renders almost ineffective any pharmacological treatment of human neoplasia.17 Thus, the identification of a mechanism that can weaken the apoptosis-resistance strategy of cancer cells is imperative to unravel novel drug targets for the design of more effective and target-selective therapeutic strategies. For instance, in an in vivo mouse xenograft study recently reported by Lin, co-administration of the anti-oxidant agent resveratrol, and conventional chemotherapy reduces brain tumour volumes by inducing cell apoptosis.37
With this perspective, the increased apoptosis of glioma cells, reported here by us, related to the GFER decreased expression, could constitute an important and sufficient biological event to improve the effectiveness of the conventional, sometimes inefficient, chemotropic agents.
In addition, the present data, together with our previous observations10, 12 and the data present in the literature,11, 13, 14, 15 strongly persuade us that ALR not only supports cell survival by its anti-apoptotic effect but also holds the crucial function of an anti-oxidative agent sustaining the levels of reducing agents such as clusterin. It is not easy to determine the exact sequence of these two metabolic events induced by ALR; we can only state that, at least in partially hepatectomized rats, ALR increases in the first 6–12 h after surgery, favouring the anti-apoptotic event which occurs, in the same period of the regenerative process, in the liver. More data are necessary to resolve the aspects of the physiological role of ALR.
In conclusion, the present findings suggest that ALR could be considered as a potential new target for the chemotherapic treatment of gliomas and, considering the presence of ALR in all eukaryotic cells,6, 7, 8, 9, 10, 11, 12, 13, 14 and even in the viruses38 we can hazard to say that it could be a possible support for the treatment of all human neoplasia. To our knowledge, with the data present in the literature, we cannot consider the ALR as an oncogene; we can only state that ALR is a factor overexpressed in all neoplasia so far studied8, 14 and that for the exact definition of its role in the carcinogenesis, more studies are necessary. It is interesting to note that an ALR-KO mouse strain is not yet available, probably because the downregulation of GFER is not consistent with the cell survival.
Materials and Methods
Reagents
rALR expressed in transfected COS-1 cells has been produced by the Laboratory of Biochemistry and Molecular Biology (University of Georgia, Athens, GA, USA). In line with other investigators, in the present experiments, we used a rALR form of 15 kDa, containing the well-conserved, biologically active, C-terminal domain of the molecule.29, 30, 39 All the chemical reagents were purchased, if not specifically reported, from Sigma-Aldrich (Milan, Italy). The polyclonal antibody against ALR (MultiBind GmbH, Cologne, Germany; a gift from Dr. Thomas Lisowsky) has already been employed for specific identification of ALR.7, 10, 12 For raising the ALR antibody, a purified hexahistidinyl-tagged carboxyl-terminal fragment of ALR (residues 81–205) was used.7
Cell line
Human-derived T98G glioma cells (Interlab Cell Line Collection, Genoa, Italy) were cultured in Dulbecco's modified Eagle's medium (DMEM) (Sigma-Aldrich) supplemented with 10% (v/v) inactivated FBS (PAA Laboratories GmbH, Pasching, Austria), 2 mM L-glutamine (Sigma-Aldrich), 100 μg/ml penicillin and 100 μg/ml streptomycin (Sigma-Aldrich,) at 37°C in 5% CO2. The cells, grown to 70% confluence and treated for 48 h, have been then trypsinized or treated as requested by the different protocol of the experiments and the biological parameters evaluated.
RNA interference
For the RNA interference experiments, the specific pre-designed siRNA (5′-GGAGUGUGCUGAAGACCUATT-3′; Applied Biosystems/Ambion, Austin, TX, USA), targeting the mRNA of the human GFER gene, was transfected, according to the manufacturer's protocol, in T98G glioma cells using siPORT NeoFX Transfection Agent (Applied Biosystems/Ambion). Silencer Negative Control 1 siRNA AM4635 (Applied Biosystems/Ambion) with no significant similarity to human, mouse or rat gene sequences was used as negative control.
H2O2-induced oxidative stress
Oxidative stress in human T98G glioma cells was caused by the addition of H2O2 (Sigma-Aldrich) to culture medium. Prior to each experiment, H2O2 was freshly prepared from 30% stock solution. To determine the most appropriate conditions for our experiments, different concentrations of H2O2 (50, 100 and 200 μM) were initially used (data not reported). Based on the data obtained using the different H2O2 concentrations in our previous experiments with neuroblastoma cells12 and on the references reported in the literature,40 in the present study, we decided to use H2O2 at a concentration of 100 μM. Culture cells maintained in DMEM supplemented with 10% (v/v) inactivated FBS and without H2O2 were used as control.
rALR treatment
With the purpose to test the ALR capacity to counteract the H2O2-induced apoptosis, rALR (from 1 to 100 ng/ml) was added to the culture medium of H2O2-treated glioma cells. The short form (15 kDa) of ALR was used. This molecule corresponds to the physiologically active protein that was originally identified and isolated by us5 and whose biological activity has been demonstrated in the majority of the experiments. Indeed, most independent scientific publications from a large number of research groups have used the 15-kDa ALR variant for their experiments4, 10, 12, 14, 15, 29 and this is now the general standard, in ALR experiments, to have comparable data. T98G cells, maintained in DMEM supplemented with 10% (v/v) inactivated FBS were used as control. The rALR treatment was done, at least, three times for each experimental protocol.
ALR expression
Western blot analysis
Human T98G glioma cells were grown in 75-cm2 flasks in DMEM supplemented with 10% FBS and in the presence (siRNA/ALR-treated cells) or absence (control cells) of GFER-specific siRNA. Cytosolic protein preparation and subsequent electrophoresis were done following a standard procedure.12 Briefly, after 48 h of incubation, 2 × 106 glioma cells were trypsinized (Trypsin 0.05%/EDTA 0.02%; M-Medical srl, Milan, Italy), added with 200 μl of RIPA buffer (Sigma-Aldrich), containing protease inhibitors (protease-inhibitor cocktail tablets 1 × , Roche Applied Science, Milan, Italy) and anti-phosphatases (sodium orthovanadate 2 mM; Sigma-Aldrich), gently stirred, centrifuged for 20 min at 14 000 × g at 4 °C and the supernatant collected. Protein concentration was evaluated using the Bradford assay (Bio-Rad Laboratories, Milan, Italy). Aliquots of 20 μg of total proteins were separated on 4–12% sodium dodecyl sulphate-polyacrylamide gels (Invitrogen srl), transferred onto a nitrocellulose membrane and then probed with primary antibody specific for ALR, diluted 1 : 200 in a blocking solution (5% of non-fat dry milk) (Bio-Rad Laboratories) in TBS-T (Tris Buffer Saline-Tween 20). The primary antibody was identified by an HRP-conjugated secondary antibody diluted 1 : 20 000 (Bio-Rad Laboratories), subsequently detected by a chemiluminescent substrate of HRP (Pierce Biotechnology, Inc., Rockford, IL, USA). To detect the β-actin housekeeping protein, the membrane was washed with the Restore Western Blot Stripping Buffer (Pierce Biotechnology, Inc.) for 15 min and reprobed with the anti-β-actin primary antibody diluted 1 : 500 (Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA), subsequently identified by an HRP-conjugated secondary antibody diluted 1 : 20 000 (Bio-Rad Laboratories) and detected by a chemiluminescent substrate of HRP (Pierce Biotechnology, Inc.) as for ALR detection. The densitometric analysis of each protein-related signal was obtained using the Molecular Imager Chemidoc (Bio-Rad Laboratories) and normalized against β-actin expression.
Confocal microscopy
Human T98G glioma cells were grown, for 48 h, onto cover glasses, maintained in petri dishes in DMEM added with 10% FBS in presence (siRNA/ALR-treated cells) or absence (control cells) of GFER-specific siRNA. The cover glasses were then pulled out from the dishes and fixed in 4% PFA (Sigma-Aldrich) in PBS for 15 min and washed with PBS. The cells were then permeabilized by 0.25% Triton X-100 (Sigma-Aldrich) for 20 min. After three washes with PBS, nonspecific protein binding was inhibited by a blocking solution (5% of FBS and 3% BSA) (Sigma-Aldrich) in PBS for 2 h. The cells were then incubated overnight at 4°C with the anti-ALR antibody diluted 1 : 200 in the blocking solution. A negative control was performed treating different cover glasses with the blocking solution short of the ALR-specific primary antibody. The cells were then washed with PBS and incubated with the Alexa Fluor 488 secondary antibody (Invitrogen srl) diluted 1 : 200 and with TO-PRO-3 (Invitrogen srl) diluted 1 : 7000 in PBS for 20 min for nuclear staining. The cover glasses were then mounted with Fluoromount K024, an anti-fading agent (Diagnostic BioSystems, Pleasanton, CA, USA), and analyzed with the confocal microscope Leica TCS SP2 (Leica Microsystems, Wetzlar, Germany). To verify the specificity of the immunoreaction, appropriate controls were performed incubating the cells with only the secondary antibody or using the pre-immune rabbit serum as primary antibody. We repeated the experiments three times. The green colour identifies ALR immunodetection and the blue colour identifies the nuclei.
Clusterin expression
Western blot analysis
Cytosolic protein preparation and subsequent protein electrophoresis were done as described for ALR western blot analysis. The nitrocellulose membrane, with the transferred proteins, were probed with a primary antibody specific for clusterin (Santa Cruz Biotechnology, Inc.) diluted 1 : 200 in blocking solution, subsequently identified by an HRP-conjugated secondary antibody (Bio-Rad Laboratories) and finally detected by a chemiluminescent substrate of HRP (Pierce Biotechnology, Inc.) and normalized to β-actin signal. Clusterin and β-actin densitometric expression was determined by the Molecular Imager Chemidoc (Bio-Rad Laboratories) as referred for ALR western blot analysis. The evaluation was done at least three times.
Confocal microscopy
Human T98G glioma-cell preparation for clusterin immunofluorescence detection was performed as described for ALR immunofluorescence detection. The cells, grown on cover glasses, were incubated with the anti-clusterin antibody (Santa Cruz Biotechnology, Inc.), diluted 1 : 200 in the blocking solution, overnight at 4°C. A negative control was performed treating different cover glasses with the blocking solution short of the clusterin-specific primary antibody. The cells were then washed with PBS and incubated with an Alexa Fluor secondary antibody (Invitrogen srl) diluted 1 : 200 and with TO-PRO-3 (Invitrogen srl) diluted 1 : 7000 in PBS for 20 min for nuclear staining. The cover glasses were then mounted with Fluoromount K024 and analyzed with the confocal microscope Leica TCS SP2 (Leica Microsystems, Wetzlar, Germany). To verify the specificity of the immunoreaction, appropriate controls were prepared, incubating the cells with only the secondary antibody or using the pre-immune rabbit serum as primary antibody. The green colour identifies clusterin immunodetection and the blue colour identifies the nuclei. We repeated the experiments at least three times.
ROS generation
ROS generation was monitored using an oxidation-sensitive fluorescent probe DCFH-DA (Sigma-Aldrich). Non-ionic, non-polar DCFH-DA crosses cell membranes and is hydrolyzed by intracellular esterases to the nonfluorescent (DCFH), which can be rapidly oxidized to the highly fluorescent 2,7-dichlorofluorescein in the presence of ROS. In all, 5 × 105 siRNA/ALR-treated or control T98G cells per well were plated into sterile black Culture Plate 96-F wells (PerkinElmer, Life and Analytical Sciences, Inc, Waltham, MA, USA). After 48 h, the cells were incubated (or not) with a final 100 μM H2O2 per well in DMEM and incubated for 10 min at 37 °C in 5% CO2. Then the cells were washed twice with PBS and incubated for 4 h at 37 °C in 5% CO2 with different concentrations of rALR (1–100 ng/ml). Subsequently, the culture medium was removed and cells were incubated with 0.1 mM DCFH-DA in DMEM at 37 °C in 5% CO2 in the dark for an additional 30 min and then washed thrice with PBS. Fluorescence intensity was measured directly in each well at an excitation wavelength of 485±20 nm and an emission wavelength of 530±25 nm using the multiwell fluorescence plate reader Wallac Victor mod.1421 (PerkinElmer).
Apoptotic gene expression
Bax, bcl-2, caspase-9 and activated caspase-3 protein expression were evaluated using western blot analysis on nitrocellulose membranes prepared as reported for ALR western blot analysis. Each nitrocellulose membrane was probed with the primary antibody specific for bax or bcl-2 (Santa Cruz Biotechnology, Inc.), or for caspase-9 (Santa Cruz Biotechnology, Inc.) or activated caspase-3 (Abcam Ltd, Cambridge, UK), all diluted to 1 : 200 in the blocking solution. The primary antibody was then identified by an HRP-conjugated secondary antibody (Bio-Rad Laboratories) diluted 1 : 20 000, which was subsequently detected by a chemiluminescent substrate of HRP (Pierce Biotechnology, Inc.). Each protein-related electrophoretic band was evaluated as referred for ALR western blot analysis and the densitometric value normalized with β-actin protein signal. Each analysis was done five times.
Flow-cytometric detection of apoptotic cells
Annexin V-FITC/7-AAD Kit PN IM3614 (Beckman Coulter, Milan, Italy) was used to detect apoptosis on siRNA/ALR-treated or control T98G cells, grown in 75-cm2 flasks in complete medium (DMEM+10% FBS). After 48 h, the cells were trypsinized (trypsin 0.05%/EDTA 0.02%) (M-Medical srl), washed with cold PBS and then centrifuged and resuspended in cold 1 × binding buffer to 5 × 106 cells/ml. Ten μl of annexin V-FITC solution and 20 μl of AAD viability dye were then added to 100 μl of the cell suspensions and the mixture was kept on ice for 15 min in the dark. Subsequently, 400 μl of 1 × binding buffer was added and gently mixed. The cell preparation was then analyzed by Cytomics FC500 (Beckman Coulter) flow cytometry within 30 min. The average of the different cell populations was determined using siRNA/ALR and control cells from, at least, three different experiments.
EM
siRNA/ALR-treated or control T98G cells, maintained in 75-cm2 flasks in complete culture medium (DMEM+10% FBS) for 48 h, were trypsinized (trypsin 0.05%/EDTA 0.02%; M-Medical srl) and then fixed in a mixture of 3% PFA and 1% glutaraldehyde in 0.1 M PBS at pH 7.4 for 5 h at 4 °C. Subsequently, the cells were postfixed in 1% OsO4 in PBS for 30 min at 4 °C, washed in several changes of PBS, dehydrated in graded alcohols and embedded in Epon-Araldite (TAAB, Reading, UK). Semi-thin sections (1 μm thick) were heat-stained with toluidine blue borate. Ultra-thin sections for EM were mounted on formvar-coated nickel grids and stained routinely with uranyl acetate and lead citrate. The grids were observed under a Morgagni 268 electron microscope (FEI, Hillsboro, OR, USA).
Statistical analysis
The results obtained are expressed as mean±S.D. Statistical comparison among groups was determined using analysis of variance. Where indicated, individual comparisons were performed using Student's t-test. Statistical significance was ascribed to the data when P<0.05. If not specifically reported, each datum is representative of at least three different and separated experiments.
Abbreviations
- ALR:
-
Augmenter of Liver Regeneration
- rALR:
-
recombinant ALR
- GFER :
-
growth factor erv1-like
- PBS:
-
phosphate buffer saline
- FBS:
-
foetal bovine serum
- ROS:
-
reactive oxygen species
- siRNA:
-
small interfering RNA
- TNFR:
-
tumour necrosis-factor receptor
- TRAIL:
-
tumour necrosis factor-related apoptosis inducing ligand receptors
- HSS:
-
hepatic stimulatory substance
- HCC:
-
hepatocellular carcinoma
- CH:
-
chronic hepatitis
- 7-AAD:
-
7-aminoactinomycin D
- EDTA:
-
ethylene diamine tetra acetate
- SDS:
-
sodium dodecyl sulphate
- IGEPAL:
-
octylphenoxypolyethoxyethanol
- IMS:
-
intermembrane space
- OXPHOS:
-
oxidative phosphorylation
- mtTFA:
-
mitochondrial transcription factor A
- PH:
-
partial hepatectomy
- PFA:
-
paraformaldehyde
- BSA:
-
bovine serum albumin
- HRP:
-
horseradish peroxidase
- DCFH-DA:
-
27-dichlorofluorescin diacetate
- LPO:
-
lipid peroxidation
- Mia40:
-
mitochondrial intermembrane space import and assembly protein 40
References
Wrensch M, Minn Y, Chew T, Bondy M, Berger MS . Epidemiology of primary brain tumors: current concepts and review of the literature. Neuro Oncol 2002; 4: 278–299.
Behin A, Hoang-Xuan K, Carpentier AF, Delattre JY . Primary brain tumours in adults. Lancet 2003; 361: 323–331.
Mercer RW, Tyler MA, Ulasov IV, Lesniak MS . Targeted therapies for malignant glioma: progress and potential. BioDrugs 2009; 23: 25–35.
Lisowsky T, Lee JE, Polimeno L, Francavilla A, Hofhaus G . Mammalian augmenter of liver regeneration protein is a sulfhydryl oxidase. Dig Liver Dis 2001; 33: 173–180.
Hagiya M, Francavilla A, Polimeno L, Ihara I, Sakai H, Seki T et al. Cloning and sequence analysis of the rat augmenter of liver regeneration (ALR) gene: expression of biologically active recombinant ALR and demonstration of tissue distribution. Proc Natl Acad Sci USA 1994; 91: 8142–8146.
Polimeno L, Lisowsky T, Francavilla A . From yeast to man--from mitochondria to liver regeneration: a new essential gene family. Ital J Gastroenterol Hepatol 1999; 31: 494–500.
Polimeno L, Pesetti B, Giorgio F, Moretti B, Resta L, Rossi R et al. Expression and localization of augmenter of liver regeneration in human muscle tissue. Int J Exp Pathol 2009; 90: 423–430.
Yu HY, Xiang DR, Huang HJ, Li J, Sheng JF . Expression level of augmenter of liver regeneration in patients with hepatic failure and hepatocellular carcinoma. Hepatobiliary Pancreat Dis Int 2010; 9: 492–498.
Yang X, Xie L, Qiu Z, Wu Z, He F . Human augmenter of liver regeneration: Molecular cloning, biological activity and roles in liver regeneration. Sci China C Life Sci 1997; 40: 642–647.
Polimeno L, Pesetti B, Annoscia E, Giorgio F, Francavilla R, Lisowsky T et al. Alrp, a survival factor that controls the apoptotic process of regenerating liver after partial hepatectomy in rats. Free Radic Res 2011; 45: 534–549.
Thirunavukkarasu C, Wang LF, Harvey SA, Watkins SC, Chaillet JR, Prelich J et al. Augmenter of liver regeneration: an important intracellular survival factor for hepatocytes. J Hepatol 2008; 48: 578–588.
Polimeno L, Pesetti B, Lisowsky T, Iannone F, Resta L, Giorgio F et al. Protective effect of augmenter of liver regeneration on hydrogen peroxide-induced apoptosis in SH-SY5Y human neuroblastoma cells. Free Radic Res 2009; 43: 865–875.
Cao Y, Fu YL, Yu M, Yue PB, Ge CH, Xu WX et al. Human augmenter of liver regeneration is important for hepatoma cell viability and resistance to radiation-induced oxidative stress. Free Radic Biol Med 2009; 47: 1057–1066.
Todd LR, Damin MN, Gomathinayagam R, Horn SR, Means AR, Sankar U et al. Growth factor erv1-like modulates Drp1 to preserve mitochondrial dynamics and function in mouse embryonic stem cells. Mol Biol Cell 2010; 21: 1225–1236.
Liao XH, Zhang L, Liu Q, Sun H, Peng CM, Guo H . Augmenter of liver regeneration protects kidneys from ischaemia/reperfusion injury in rats. Nephrol Dial Transplant 2010; 25: 2921–2929.
Noh EM, Yi MS, Youn HJ, Lee BK, Lee YR, Han JH et al. Silibinin enhances ultraviolet B-induced apoptosis in mcf-7 human breast cancer cells. J Breast Cancer 2011; 14: 8–13.
Indrani IR, Tufo G, Pervaiz S, Brenner C . Recent advances in apoptosis, mitochondria and drug resistance in cancer cells. BBA 2011; 1807: 735–745.
Hergartner MO . The biochemistry of apoptosis. Nature 2000; 407: 770–776.
Francavilla A, Hagiya M, Porter KA, Polimeno L, Ihara I, Starzl TE . Augmenter of liver regeneration: its place in the universe of hepatic growth factors. Hepatology 1994; 20: 747–757.
Francavilla A, Ove P, Polimeno L, Coetzee M, Makowka L, Rose J et al. Extraction and partial purification of a hepatic stimulatory substance in rats, mice, and dogs. Cancer Res. 1987; 47: 5600–5605.
Francavilla A, Starzl TE, Porter K, Foglieni CS, Michalopoulos GK, Carrieri G et al. Screening for candidate hepatic growth factors by selective portal infusion after canine Eck's fistula. Hepatology 1991; 14: 665–670.
Sakamoto T, Liu Z, Murase N, Ezure T, Yokomuro S, Poli V et al. Mitosis and apoptosis in the liver of interleukin-6-deficient mice after partial hepatectomy. Hepatology 1999; 29: 403–411.
Li Y, Wei K, Lu C, Li Y, Li M, Xing G et al. Identification of hepatopoietin dimerizzation, its interacting regions and alternative splicing of its transcript. Eur J Biochem 2002; 269: 3888–3893.
Wu CK, Dailey TA, Dailey HA, Wang BC, Rose JP . The cristal structure of Augmenter of liver regeneration: a mammalian FAD-dependent sulfhydryl oxidase. Protein Sci 2003; 12: 1109–1118.
Farrell SR, Thorpe C . Augmenter of liver regeneration: A flavin-dependent sulfhydryl oxidase with cytochrome c reductase activity. Biochemestry 2005; 44: 1532–1541.
Banci L, Bertini I, Calderone V, Cefaro C, Ciofi-Baffoni S, Gallo A et al. Molecular chaperone function of Mia40 triggers consecutive induced folding steps of the substrate in mitochondrial protein import. Proc Natl Acad Sci USA 2010; 107: 20190–20195.
Lange H, Lisowsky T, Gerber J, Mühlenhoff U, Kispal G, Lill R . An essential function of the mitochondrial sulfhydryl oxidase Erv1p/ALR in the maturation of cytosolic Fe/S proteins. EMBO Rep 2001; 2: 715–720.
Djeu JY, Wei S . Clusterin and chemoresistance. Adv Cancer Res 2009; 105: 77–92.
Gao CF, Zhou FG, Wang H, Huang YF, Ji Q, Chen J . Genetic recombinant expression and characterization of human augmenter of liver regeneration. Dig Dis Sci 2009; 54: 530–537.
Polimeno L, Capuano F, Marangi LC, Margiotta M, Lisowsky T, Ierardi E et al. The augmenter of liver regeneration induces mitochondrial gene expression in rat liver and enhances oxidative phosphorylation capacity of liver mitochondria. Dig Liver Dis 2000; 32: 510–517.
Dewaele M, Maes H, Agostinis P . ROS-mediated mechanisms of autophagy stimulation and their relevance in cancer therapy. Autophagy 2010; 6: 838–854.
Klaunig JE, Kamendulis LM, Hocevar BA . Oxidative stress and oxidative damage in carcinogenesis. Toxicol Pathol 2010; 38: 96–109.
Fruehauf JP, Meyskens Jr FL . Reactive Oxygen Species: A Breath of Life or Death? Clin Cancer Res 2007; 13: 789–794.
Konturek PC, Konturek SJ, Brzozowski T . Gastric cancer and Helicobacter pylori infection. J Physiol Pharmacol 2006; 57 (Suppl 3): 51–65.
Goldman A, Chen H, Khan MR, Roesly H, Hill KA, Shahidullah M et al. The Na/H exchanger controls deoxycholic Acid-induced apoptosis by a h-activated, Na-dependent ionic shift in esophageal cells. PLoS One 2011; 6: e23835.
Guan H, Zhang H, Cai J, Wu J, Yuan J, Li J et al. IKBKE is over-expressed in glioma and contributes to resistance of glioma cells to apoptosis via activating NF-κB. J Pathol 2011; 223: 436–445.
Lin CJ, Lee CC, Shih YL, Lin TY, Wang SH, Lin YF et al. Resveratrol enhances the therapeutic effect of temozolomide against malignant glioma in vitro and in vivo by inhibiting autophagy. Free Radic Biol Med 2011; 52: 377–391.
Senkevich TG, White CL, Koonin EV, Moss B . A viral member of the ERV1/ALR protein family participates in a cytoplasmic pathway of disulfide bond formation. Proc Natl Acad Sci USA 2000; 97: 12068–12073.
Francavilla A, Vujanovic NL, Polimeno L, Azzarone A, Iacobellis A, Deleo A et al. The in vivo effect of hepatotrophic factors augmenter of liver regeneration, hepatocyte growth factor, and insulin-like growth factor-II on liver natural killer cell functions. Hepatology 1997; 25: 411–415.
Zhang L, Yu H, Sun Y, Lin X, Chen B, Tan C et al. Protective effects of salidroside on hydrogen peroxide-induced apoptosis in SH-SY5Y human neuroblastoma cells. Eur J Pharmacol 2007; 564: 18–25.
Acknowledgements
We are grateful to Dr. Thomas Lisowsky for his help for the final draft of the manuscript. This study was financially supported by grants from the University of Bari ‘Aldo Moro’, Bari, Italy, and from IRCCS ‘De Bellis’, Castellana Grotte, Bari, Italy.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Edited by A Stephanou
Rights and permissions
This work is licensed under the Creative Commons Attribution-NonCommercial-No Derivative Works 3.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-nd/3.0/
About this article
Cite this article
Polimeno, L., Pesetti, B., De Santis, F. et al. Decreased expression of the Augmenter of Liver Regeneration results in increased apoptosis and oxidative damage in human-derived glioma cells. Cell Death Dis 3, e289 (2012). https://doi.org/10.1038/cddis.2012.25
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/cddis.2012.25
Keywords
This article is cited by
-
Polymorphism rs1046495 of the GFER Gene as a New Genetic Marker of Preposition to Type 2 Diabetes Mellitus
Bulletin of Experimental Biology and Medicine (2022)
-
Augmenter of liver regeneration regulates autophagy in renal ischemia–reperfusion injury via the AMPK/mTOR pathway
Apoptosis (2017)
-
Augmenter of liver regeneration plays a protective role against hydrogen peroxide-induced oxidative stress in renal proximal tubule cells
Apoptosis (2015)
-
Common Players in Mitochondria Biogenesis and Neuronal Protection Against Stress-Induced Apoptosis
Neurochemical Research (2014)