Abstract
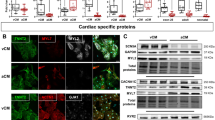
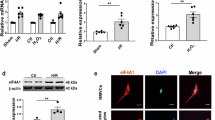
Although myocyte cell transplantation studies have suggested a promising therapeutic potential for myocardial infarction, a major obstacle to the development of clinical therapies for myocardial repair is the difficulties associated with obtaining relatively homogeneous ventricular myocytes for transplantation. Human embryonic stem cells (hESCs) are a promising source of cardiomyocytes. Here we report that retinoid signaling regulates the fate specification of atrial versus ventricular myocytes during cardiac differentiation of hESCs. We found that both Noggin and the pan-retinoic acid receptor antagonist BMS-189453 (RAi) significantly increased the cardiac differentiation efficiency of hESCs. To investigate retinoid functions, we compared Noggin+RAi-treated cultures with Noggin+RA-treated cultures. Our results showed that the expression levels of the ventricular-specific gene IRX-4 were radically elevated in Noggin+RAi-treated cultures. MLC-2V, another ventricular-specific marker, was expressed in the majority of the cardiomyocytes in Noggin+RAi-treated cultures, but not in the cardiomyocytes of Noggin+RA-treated cultures. Flow cytometry analysis and electrophysiological studies indicated that with 64.7 ± 0.88% (mean ±s.e.m) cardiac differentiation efficiency, 83% of the cardiomyocytes in Noggin+RAi-treated cultures had embryonic ventricular-like action potentials (APs). With 50.7 ± 1.76% cardiac differentiation efficiency, 94% of the cardiomyocytes in Noggin+RA-treated cultures had embryonic atrial-like APs. These results were further confirmed by imaging studies that assessed the patterns and properties of the Ca2+ sparks of the cardiomyocytes from the two cultures. These findings demonstrate that retinoid signaling specifies the atrial versus ventricular differentiation of hESCs. This study also shows that relatively homogeneous embryonic atrial- and ventricular-like myocyte populations can be efficiently derived from hESCs by specifically regulating Noggin and retinoid signals.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
He JQ, Ma Y, Lee Y, Thomson JA, Kamp TJ . Human embryonic stem cells develop into multiple types of cardiac myocytes: action potential characterization. Circ Res 2003; 93:32–39.
Laflamme MA, Chen KY, Naumova AV, et al. Cardiomyocytes derived from human embryonic stem cells in pro-survival factors enhance function of infarcted rat hearts. Nat Biotechnol 2007; 25:1015–1024.
Yang L, Soonpaa MH, Adler ED, et al. Human cardiovascular progenitor cells develop from a KDR+ embryonic-stem-cell-derived population. Nature 2008; 453:524–528.
Zhu WZ, Xie Y, Moyes KW, et al. Neuregulin/ErbB signaling regulates cardiac subtype specification in differentiating human embryonic stem cells. Circ Res 2010; 107:776–786.
Kehat I, Khimovich L, Caspi O, et al. Electromechanical integration of cardiomyocytes derived from human embryonic stem cells. Nat Biotechnol 2004; 22:1282–1289.
Chen HS, Kim C, Mercola M . Electrophysiological challenges of cell-based myocardial repair. Circulation 2009; 120:2496–2508.
Tran TH, Wang X, Browne C, et al. Wnt3a-induced mesoderm formation and cardiomyogenesis in human embryonic stem cells. Stem Cells 2009; 27: 1869–1878.
Reppel M, Igelmund P, Egert U, et al. Effect of cardioactive drugs on action potential generation and propagation in embryonic stem cell-derived cardiomyocytes. Cell Physiol Biochem 2007; 19:213–224.
Yuasa S, Itabashi Y, Koshimizu U, et al. Transient inhibition of BMP signaling by Noggin induces cardiomyocyte differentiation of mouse embryonic stem cells. Nat Biotechnol 2005; 23:607–611.
Marvin MJ, Di Rocco G, Gardiner A, Bush SM, Lassar AB . Inhibition of Wnt activity induces heart formation from posterior mesoderm. Genes Dev 2001; 15:316–327.
Schneider VA, Mercola M . Wnt antagonism initiates cardiogenesis in Xenopus laevis. Genes Dev 2001; 15:304–315.
Korol O, Gupta RW, Mercola M . A novel activity of the Dickkopf-1 amino terminal domain promotes axial and heart development independently of canonical Wnt inhibition. Dev Biol 2008; 324:131–138.
Xu RH, Chen X, Li DS, et al. BMP4 initiates human embryonic stem cell differentiation to trophoblast. Nat Biotechnol 2002; 20:1261–1264.
Zhang P, Li J, Tan Z, et al. Short-term BMP-4 treatment initiates mesoderm induction in human embryonic stem cells. Blood 2008; 111:1933–1941.
Schulze GE, Clay RJ, Mezza LE, et al. BMS-189453, a novel retinoid receptor antagonist, is a potent testicular toxin. Toxicol Sci 2001; 59:297–308.
Keegan BR, Feldman JL, Begemann G, Ingham PW, Yelon D . Retinoic acid signaling restricts the cardiac progenitor pool. Science 2005; 307:247–249.
Xavier-Neto J, Rosenthal N, Silva FA, et al. Retinoid signaling and cardiac anteroposterior segmentation. Genesis 2001; 31:97–104.
Patwardhan V, Fernandez S, Montgomery M, Litvin J . The rostro-caudal position of cardiac myocytes affect their fate. Dev Dyn 2000; 218:123–135.
Orts-Llorca F JCJ . Determination of heart polarity (arterio venous axis) in the chicken embryo. Roux Arch Entwick-lungsmechanik 1967; 113:17.
Yutzey K, Gannon M, Bader D . Diversification of cardiomyogenic cell lineages in vitro. Dev Biol 1995; 170:531–541.
Hochgreb T, Linhares VL, Menezes DC, et al. A caudorostral wave of RALDH2 conveys anteroposterior information to the cardiac field. Development 2003; 130:5363–5374.
Xavier-Neto J, Neville CM, Shapiro MD, et al. A retinoic acid-inducible transgenic marker of sino-atrial development in the mouse heart. Development 1999; 126:2677–2687.
Gassanov N, Er F, Zagidullin N, et al. Retinoid acid-induced effects on atrial and pacemaker cell differentiation and expression of cardiac ion channels. Differentiation 2008; 76:971–980.
Bao ZZ, Bruneau BG, Seidman JG, Seidman CE, Cepko CL . Regulation of chamber-specific gene expression in the developing heart by Irx4. Science 1999; 283:1161–1164.
Fu JD, Jiang P, Rushing S, et al. Na+/Ca2+ exchanger is a determinant of excitation-contraction coupling in human embryonic stem cell-derived ventricular cardiomyocytes. Stem Cells Dev 2010; 19:773–782.
Maltsev VA, Rohwedel J, Hescheler J, Wobus AM . Embryonic stem cells differentiate in vitro into cardiomyocytes representing sinusnodal, atrial and ventricular cell types. Mech Dev 1993; 44:41–50.
Cheng H, Lederer WJ . Calcium sparks. Physiol Rev 2008; 88:1491–1545.
Woo SH, Cleemann L, Morad M . Spatiotemporal characteristics of junctional and nonjunctional focal Ca2+ release in rat atrial myocytes. Circ Res 2003; 92:e1–e11.
Cleemann L, Wang W, Morad M . Two-dimensional confocal images of organization, density, and gating of focal Ca2+ release sites in rat cardiac myocytes. Proc Natl Acad Sci USA 1998; 95:10984–10989.
Shimoji K, Yuasa S, Onizuka T, et al. G-CSF promotes the proliferation of developing cardiomyocytes in vivo and in derivation from ESCs and iPSCs. Cell Stem Cell 2010; 6:227–237.
Xu RH, Peck RM, Li DS, et al. Basic FGF and suppression of BMP signaling sustain undifferentiated proliferation of human ES cells. Nat Methods 2005; 2:185–190.
Ying QL, Nichols J, Chambers I, Smith A . BMP induction of Id proteins suppresses differentiation and sustains embryonic stem cell self-renewal in collaboration with STAT3. Cell 2003; 115:281–292.
Wobus AM, Kaomei G, Shan J, et al. Retinoic acid accelerates embryonic stem cell-derived cardiac differentiation and enhances development of ventricular cardiomyocytes. J Mol Cell Cardiol 1997; 29:1525–1539.
Domian IJ, Chiravuri M, van der Meer P, et al. Generation of functional ventricular heart muscle from mouse ventricular progenitor cells. Science 2009; 326:426–429.
Takahashi K, Tanabe K, Ohnuki M, et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell 2007; 131:861–872.
Yu J, Vodyanik MA, Smuga-Otto K, et al. Induced pluripotent stem cell lines derived from human somatic cells. Science 2007; 318:1917–1920.
Xu C, Inokuma MS, Denham J, et al. Feeder-free growth of undifferentiated human embryonic stem cells. Nat Biotech 2001; 19:971–974.
Picht E, Zima AV, Blatter LA, Bers DM . SparkMaster: automated calcium spark analysis with ImageJ. Am J Physiol Cell Physiol 2007; 293:C1073–C1081.
Acknowledgements
We thank Drs Joy Fleming and Juanjuan Feng of the Institute of Biophysics, and Dr Paul Bornstein of the University of Washington for critical discussion and revision of the manuscript, and Dr Shigang He of the Institute of Biophysics for support and access to patch clamp technology. This work was supported by the Hi-Tech Research and Development Program of China (863 Program) (2006AA02A106), the National Basic Research Program of China (2006CB943901, 2010CB945024, and 2011CB965002), the Knowledge Innovation Program of the Chinese Academy of Sciences (KSCX2-YW-R-50), and the National Foundation of Science and Technology (30640005).
Author information
Authors and Affiliations
Corresponding authors
Additional information
( Supplementary information is linked to the online version of the paper on the Cell Research website.)
Supplementary information
Supplementary information, Movie S1
shows contracting clusters in 60-day-old differentiated hESC cultures treated with Ngn+RA. (MOV 3977 kb)
Supplementary information, Movie S2
shows contracting clusters in 60-day-old differentiated hESC cultures treated with Ngn+RAi. (MOV 4364 kb)
Supplementary information, Figure S1
MLC-2V expression in different retinoid-treated cultures. (PDF 1625 kb)
Supplementary information, Figure S2
Cardiac gene expression in differently treated cultures. (PDF 171 kb)
Rights and permissions
About this article
Cite this article
Zhang, Q., Jiang, J., Han, P. et al. Direct differentiation of atrial and ventricular myocytes from human embryonic stem cells by alternating retinoid signals. Cell Res 21, 579–587 (2011). https://doi.org/10.1038/cr.2010.163
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/cr.2010.163
Keywords
This article is cited by
-
In situ monolayer patch clamp of acutely stimulated human iPSC-derived cardiomyocytes promotes consistent electrophysiological responses to SK channel inhibition
Scientific Reports (2024)
-
JAK2 as a surface marker for enrichment of human pluripotent stem cells-derived ventricular cardiomyocytes
Stem Cell Research & Therapy (2023)
-
Biophysical properties of NaV1.5 channels from atrial-like and ventricular-like cardiomyocytes derived from human induced pluripotent stem cells
Scientific Reports (2023)
-
Retinoic acid signaling modulation guides in vitro specification of human heart field-specific progenitor pools
Nature Communications (2023)
-
Long-term in vitro recording of cardiac action potentials on microelectrode arrays for chronic cardiotoxicity assessment
Archives of Toxicology (2023)