Abstract
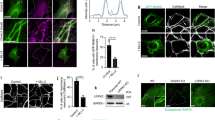
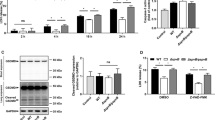
Salmonella can invade non-phagocytic cells through its type III secretion system (T3SS-1), which induces a Trigger entry process. This study showed that Salmonella enterica, subspecies enterica serovar Enteritidis can also invade cells via the Rck outer membrane protein. Rck was necessary and sufficient to enable non-invasive E. coli and Rck-coated beads to adhere to and invade different cells. Internalization analysis of latex beads coated with different Rck peptides showed that the peptide containing amino acids 140-150 promoted adhesion, whereas amino acids between 150 and 159 modulated invasion. Expression of dominant-negative derivatives and use of specific inhibitors demonstrated the crucial role of small GTPases Rac1 and Cdc42 in activating the Arp2/3 complex to trigger formation of actin-rich accumulation, leading to Rck-dependent internalization. Finally, scanning and transmission electron microscopy with Rck-coated beads and E. coli expressing Rck revealed microvillus-like extensions that formed a Zipper-like structure, engulfing the adherent beads and bacteria. Overall, our results provide new insights into the Salmonella T3SS-independent invasion mechanisms and strongly suggest that Rck induces a Zipper-like entry mechanism. Consequently, Salmonella seems to be the first bacterium found to be able to induce both Zipper and Trigger mechanisms to invade host cells.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Seveau S, Pizarro-Cerda J, Cossart P . Molecular mechanisms exploited by Listeria monocytogenes during host cell invasion. Microbes Infect 2007; 9:1167–1175.
Cossart P, Sansonetti PJ . Bacterial invasion: the paradigms of enteroinvasive pathogens. Science 2004; 304:242–248.
Wiedemann A, Linder S, Grassl G, Albert M, Autenrieth I, Aepfelbacher M . Yersinia enterocolitica invasin triggers phagocytosis via beta1 integrins, CDC42Hs and WASp in macrophages. Cell Microbiol 2001; 3:693–702.
Schlumberger MC, Hardt WD . Salmonella type III secretion effectors: pulling the host cell's strings. Curr Opin Microbiol 2006; 9:46–54.
Waterman SR, Holden DW . Functions and effectors of the Salmonella pathogenicity island 2 type III secretion system. Cell Microbiol 2003; 5:501–511.
Heffernan EJ, Wu L, Louie J, Okamoto S, Fierer J, Guiney DG . Specificity of the complement resistance and cell association phenotypes encoded by the outer membrane protein genes rck from Salmonella Typhimurium and ail from Yersinia enterocolitica. Infect Immun 1994; 62:5183–5186.
Lambert MA, Smith SG . The PagN protein of Salmonella enterica serovar Typhimurium is an adhesin and invasin. BMC Microbiol 2008; 8:142.
Lambert MA, Smith SG . The PagN protein mediates invasion via interaction with proteoglycan. FEMS Microbiol Lett 2009; 297:209–216.
Heffernan EJ, Reed S, Hackett J, Fierer J, Roudier C, Guiney D . Mechanism of resistance to complement-mediated killing of bacteria encoded by the Salmonella Typhimurium virulence plasmid gene rck. J Clin Invest 1992; 90:953–964.
Taylor PW . Bactericidal and bacteriolytic activity of serum against gram-negative bacteria. Microbiol Rev 1983; 47:46–83.
Heffernan EJ, Harwood J, Fierer J, Guiney D . The Salmonella Typhimurium virulence plasmid complement resistance gene rck is homologous to a family of virulence-related outer membrane protein genes, including pagC and ail. J Bacteriol 1992; 174:84–91.
Ishidate K, Creeger ES, Zrike J, et al. Isolation of differentiated membrane domains from Escherichia coli and Salmonella Typhimurium, including a fraction containing attachment sites between the inner and outer membranes and the murein skeleton of the cell envelope. J Biol Chem 1986; 261:428–443.
Schnaitman CA . Protein composition of the cell wall and cytoplasmic membrane of Escherichia coli. J Bacteriol 1970; 104:890–901.
Smith JN, Ahmer BM . Detection of other microbial species by Salmonella: expression of the SdiA regulon. J Bacteriol 2003; 185:1357–1366.
Kim W, Surette MG . Coordinated regulation of two independent cell-cell signaling systems and swarmer differentiation in Salmonella enterica serovar Typhimurium. J Bacteriol 2006; 188:431–440.
Cooper JA . Effects of cytochalasin and phalloidin on actin. J Cell Biol 1987; 105:1473–1478.
Cirillo DM, Heffernan EJ, Wu L, Harwood J, Fierer J, Guiney DG . Identification of a domain in Rck, a product of the Salmonella Typhimurium virulence plasmid, required for both serum resistance and cell invasion. Infect Immun 1996; 64:2019–2023.
Mengaud J, Ohayon H, Gounon P, Mege RM, Cossart P . E-cadherin is the receptor for internalin, a surface protein required for entry of L. monocytogenes into epithelial cells. Cell 1996; 84:923–932.
Millard TH, Sharp SJ, Machesky LM . Signalling to actin assembly via the WASP (Wiskott-Aldrich syndrome protein)-family proteins and the Arp2/3 complex. Biochem J 2004; 380:1–17.
Sousa S, Cabanes D, Bougneres L, et al. Src, cortactin and Arp2/3 complex are required for E-cadherin-mediated internalization of Listeria into cells. Cell Microbiol 2007; 9:2629–2643.
Machesky LM, Insall RH . Scar1 and the related Wiskott-Aldrich syndrome protein, WASP, regulate the actin cytoskeleton through the Arp2/3 complex. Curr Biol 1998; 8:1347–1356.
Andor A, Trulzsch K, Essler M, et al. YopE of Yersinia, a GAP for Rho GTPases, selectively modulates Rac-dependent actin structures in endothelial cells. Cell Microbiol 2001; 3:301–310.
Aktories K, Hall A . Botulinum ADP-ribosyltransferase C3: a new tool to study low molecular weight GTP-binding proteins. Trends Pharmacol Sci 1989; 10:415–418.
Ahmer BM, van Reeuwijk J, Timmers CD, Valentine PJ, Heffron F . Salmonella Typhimurium encodes an SdiA homolog, a putative quorum sensor of the LuxR family, that regulates genes on the virulence plasmid. J Bacteriol 1998; 180:1185–1193.
Sircili MP, Walters M, Trabulsi LR, Sperandio V . Modulation of enteropathogenic Escherichia coli virulence by quorum sensing. Infect Immun 2004; 72:2329–2337.
Dyszel JL, Smith JN, Lucas DE, et al. Salmonella enterica serovar Typhimurium can detect the acyl homoserine lactone production of Yersinia enterocolitica in mice. J Bacteriol 2010; 192:29–37.
Smith JN, Dyszel JL, Soares JA, et al. SdiA, an N-acylhomoserine lactone receptor, becomes active during the transit of Salmonella enterica through the gastrointestinal tract of turtles. PLoS ONE 2008; 3:e2826.
Akeda Y, Kodama T, Kashimoto T, et al. Dominant-negative Rho, Rac, and Cdc42 facilitate the invasion process of Vibrio parahaemolyticus into Caco-2 cells. Infect Immun 2002; 70:970–973.
Burnham CA, Shokoples SE, Tyrrell GJ . Rac1, RhoA, and Cdc42 participate in HeLa cell invasion by group B streptococcus. FEMS Microbiol Lett 2007; 272:8–14.
Carabeo RA, Grieshaber SS, Hasenkrug A, Dooley C, Hackstadt T . Requirement for the Rac GTPase in Chlamydia trachomatis invasion of non-phagocytic cells. Traffic 2004; 5:418–425.
Atre AN, Surve SV, Shouche YS, Joseph J, Patole MS, Deopurkar RL . Association of small Rho GTPases and actin ring formation in epithelial cells during the invasion by Candida albicans. FEMS Immunol Med Microbiol 2009; 55:74–84.
Chen LM, Hobbie S, Galan JE . Requirement of CDC42 for Salmonella-induced cytoskeletal and nuclear responses. Science 1996; 274:2115–2118.
Hardt WD, Chen LM, Schuebel KE, Bustelo XR, Galan JE . S. Typhimurium encodes an activator of Rho GTPases that induces membrane ruffling and nuclear responses in host cells. Cell 1998; 93:815–826.
Shibata T, Takeshima F, Chen F, Alt FW, Snapper SB . Cdc42 facilitates invasion but not the actin-based motility of Shigella. Curr Biol 2002; 12:341–345.
Friebel A, Ilchmann H, Aepfelbacher M, Ehrbar K, Machleidt W, Hardt WD . SopE and SopE2 from Salmonella Typhimurium activate different sets of RhoGTPases of the host cell. J Biol Chem 2001; 276:34035–34040.
Hanisch J, Ehinger J, Ladwein M, et al. Molecular dissection of Salmonella-induced membrane ruffling versus invasion. Cell Microbiol 2009; 12:84–98.
Unsworth KE, Way M, McNiven M, Machesky L, Holden DW . Analysis of the mechanisms of Salmonella-induced actin assembly during invasion of host cells and intracellular replication. Cell Microbiol 2004; 6:1041–1055.
Kieda C, Paprocka M, Krawczenko A, et al. New human microvascular endothelial cell lines with specific adhesion molecules phenotypes. Endothelium 2002; 9:247–261.
Wiedemann A, Patel JC, Lim J, Tsun A, van Kooyk Y, Caron E . Two distinct cytoplasmic regions of the beta2 integrin chain regulate RhoA function during phagocytosis. J Cell Biol 2006; 172:1069–1079.
Allen-Vercoe E, Dibb-Fuller M, Thorns CJ, Woodward MJ . SEF17 fimbriae are essential for the convoluted colonial morphology of Salmonella Enteritidis. FEMS Microbiol Lett 1997; 153:33–42.
Cougoule C, Hoshino S, Dart A, Lim J, Caron E . Dissociation of recruitment and activation of the small G-protein Rac during Fcgamma receptor-mediated phagocytosis. J Biol Chem 2006; 281:8756–8764.
Sebbagh M, Renvoize C, Hamelin J, Riche N, Bertoglio J, Breard J . Caspase-3-mediated cleavage of ROCK I induces MLC phosphorylation and apoptotic membrane blebbing. Nat Cell Biol 2001; 3:346–352.
Fardini Y, Chettab K, Grepinet O, et al. The YfgL lipoprotein is essential for type III secretion system expression and virulence of Salmonella enterica Serovar Enteritidis. Infect Immun 2007; 75:358–370.
Figueroa-Bossi N, Ammendola S, Bossi L . Differences in gene expression levels and in enzymatic qualities account for the uneven contribution of superoxide dismutases SodCI and SodCII to pathogenicity in Salmonella enterica. Microbes Infect 2006; 8:1569–1578.
Velge P, Bottreau E, Kaeffer B, Yurdusev N, Pardon P, Van Langendonck N . Protein tyrosine kinase inhibitors block the entries of Listeria monocytogenes and Listeria ivanovii into epithelial cells. Microb Pathog 1994; 17:37–50.
Patient R, Hourioux C, Sizaret PY, Trassard S, Sureau C, Roingeard P . Hepatitis B virus subviral envelope particle morphogenesis and intracellular trafficking. J Virol 2007; 81:3842–3851.
Acknowledgements
This work was supported by the Région Centre and performed within the 'SAVIRE' project, granted by the Délégation Régionale à la Recherche et à la Technologie du Centre (FEDER) (No 1634-32245) and by the Région Centre (No 2008-00036085). M Rosselin holds a Doctoral fellowship granted by the Région Centre and the Institut National de la Recherche Agronomique. We thank C Kieda for HBrMEC cell donation, Koreaki Ito (Kyoto University, Kyoto, Japan) for anti-SecG antibody donation, P Cossart and J Bertoglio for plasmid donation and J De Rycke for his critical reading of the manuscript.
Emmanuelle Caron passed away on July 8, 2009. We would like to take the opportunity to thank her for her guidance and suppport over the years. We will miss the friend and the great scientist!
Author information
Authors and Affiliations
Corresponding author
Additional information
(Supplementary information is linked to the online version of the paper on the Cell Research website.)
Supplementary information
Supplementary information, Figure S1
Rck is able to induce adhesion to and internalization of a non invasive E. coli strain by human cells. (PDF 54 kb)
Supplementary information, Figure S2
rck deletion did not significantly reduce adhesion to and internalization of S. Enteritidis into MA104 and 3T3 cell lines. (PDF 82 kb)
Supplementary information, Figure S3
GST-113-159 Rck-coated beads are internalized by MA104 cells. (PDF 138 kb)
Supplementary information, Figure S4
Rck mediates a Zipper-like membrane rearrangement. (PDF 158 kb)
Supplementary information, Figure S5
The transduced TAT-N17Rac and -N17Cdc42 fusion proteins are active. (PDF 76 kb)
Supplementary information, Figure S6
G118D amino acid substitution in Rck inhibited adhesion and cell internalization. (PDF 37 kb)
Rights and permissions
About this article
Cite this article
Rosselin, M., Virlogeux-Payant, I., Roy, C. et al. Rck of Salmonella enterica, subspecies enterica serovar Enteritidis, mediates Zipper-like internalization. Cell Res 20, 647–664 (2010). https://doi.org/10.1038/cr.2010.45
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/cr.2010.45
Keywords
This article is cited by
-
Current Progress and Challenges in the Study of Adjuvants for Oral Vaccines
BioDrugs (2023)
-
Investigation of the invasion mechanism mediated by the outer membrane protein PagN of Salmonella Typhimurium
BMC Microbiology (2021)
-
Murine AML12 hepatocytes allow Salmonella Typhimurium T3SS1-independent invasion and intracellular fate
Scientific Reports (2021)
-
Neutralizing-antibody-mediated protection of chickens against infectious bursal disease via one-time vaccination with inactivated recombinant Lactococcus lactis expressing a fusion protein constructed from the RCK protein of Salmonella enterica and VP2 of infectious bursal disease virus
Microbial Cell Factories (2019)
-
Inhibition of Salmonella Typhimurium adhesion, invasion, and intracellular survival via treatment with methyl gallate alone and in combination with marbofloxacin
Veterinary Research (2018)