Abstract
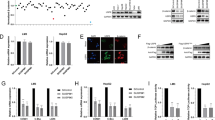
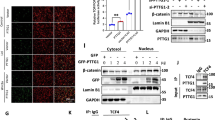
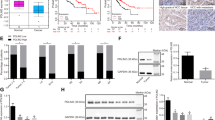
p28GANK (also known as PSMD10 or gankyrin) is a novel oncoprotein that is highly expressed in hepatocellular carcinoma (HCC). Through its interaction with various proteins, p28GANK mediates the degradation of the tumor suppressor proteins Rb and p53. Although p53 was reported to downregulate β-catenin, whether p28GANK is involved in the regulation of β-catenin remains uncertain. Here we report that both growth factors and Ras upregulate p28GANK expression through the activation of the phosphoinositide 3-kinase-AKT pathway. Upregulation of p28GANK expression subsequently enhanced the transcription activity of β-catenin. This effect was observed in p53-deficient cells, suggesting a p53-independent mechanism for the p28GANK-mediated activation of β-catenin. p28GANK overexpression also reduced E-cadherin protein levels, leading to increased release of free β-catenin into the cytoplasm from the cadherin-bound pool. Interestingly, exogenous expression of p28GANK resulted in elevated expression of the endogenous protein. We also observed that both β-catenin and c-Myc were transcriptional activators of p28GANK, and a correlation between p28GANK overexpression and c-Myc, cyclin D1 and β-catenin activation in primary human HCC. Together, these results suggest that p28GANK expression is regulated by a positive feedback loop involving β-catenin, which may play a critical role in tumorigenesis and the progression of HCC.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Befeler AS, Di Bisceglie AM . Hepatocellular carcinoma: diagnosis and treatment. Gastroenterology 2002; 122:1609–1619.
Laurent-Puig P, Zucman-Rossi J . Genetics of hepatocellular tumors. Oncogene 2006; 25:3778–3786.
Luo J, Manning BD, Cantley LC . Targeting the PI3K-Akt pathway in human cancer: rationale and promise. Cancer Cell 2003; 4:257–262.
McCormick F . Signalling networks that cause cancer. Trends Cell Biol 1999; 9:M53–M56.
Bader AG, Kang S, Zhao L, Vogt PK . Oncogenic PI3K deregulates transcription and translation. Nat Rev Cancer 2005; 5:921–929.
Fresno Vara JA, Casado E, de CJ, Cejas P, Belda-Iniesta C, Gonzalez-Baron M . PI3K/Akt signalling pathway and cancer. Cancer Treat Rev 2004; 30:193–204.
Frame S, Cohen P . GSK3 takes centre stage more than 20 years after its discovery. Biochem J 2001; 359:1–16.
Reya T, Clevers H . Wnt signalling in stem cells and cancer. Nature 2005; 434:843–850.
Jamora C, Fuchs E . Intercellular adhesion, signalling and the cytoskeleton. Nat Cell Biol 2002; 4:E101–E108.
Giles RH, van Es JH, Clevers H . Caught up in a Wnt storm: Wnt signaling in cancer. Biochim Biophys Acta 2003; 1653:1–24.
Polakis P . The oncogenic activation of beta-catenin. Curr Opin Genet Dev 1999; 9:15–21.
Taipale J, Beachy PA . The Hedgehog and Wnt signalling pathways in cancer. Nature 2001; 411:349–354.
de La CA, Romagnolo B, Billuart P, et al. Somatic mutations of the beta-catenin gene are frequent in mouse and human hepatocellular carcinomas. Proc Natl Acad Sci USA 1998; 95:8847–8851.
Suzuki T, Yano H, Nakashima Y, Nakashima O, Kojiro M . Beta-catenin expression in hepatocellular carcinoma: a possible participation of beta-catenin in the dedifferentiation process. J Gastroenterol Hepatol 2002; 17:994–1000.
Wong CM, Fan ST, Ng IO . beta-Catenin mutation and overexpression in hepatocellular carcinoma: clinicopathologic and prognostic significance. Cancer 2001; 92:136–145.
Taniguchi K, Roberts LR, Aderca IN, et al. Mutational spectrum of beta-catenin, AXIN1, and AXIN2 in hepatocellular carcinomas and hepatoblastomas. Oncogene 2002; 21:4863–4871.
Satoh S, Daigo Y, Furukawa Y, et al. AXIN1 mutations in hepatocellular carcinomas, and growth suppression in cancer cells by virus-mediated transfer of AXIN1. Nat Genet 2000; 24:245–250.
Higashitsuji H, Itoh K, Nagao T, et al. Reduced stability of retinoblastoma protein by gankyrin, an oncogenic ankyrin-repeat protein overexpressed in hepatomas. Nat Med 2000; 6:96–99.
Hori T, Kato S, Saeki M, et al. cDNA cloning and functional analysis of p28 (Nas6p) and p40.5 (Nas7p), two novel regulatory subunits of the 26S proteasome. Gene 1998; 216:113–122.
Dawson S, Apcher S, Mee M, et al. Gankyrin is an ankyrin-repeat oncoprotein that interacts with CDK4 kinase and the S6 ATPase of the 26 S proteasome. J Biol Chem 2002; 277:10893–10902.
Higashitsuji H, Higashitsuji H, Itoh K, et al. The oncoprotein gankyrin binds to MDM2/HDM2, enhancing ubiquitylation and degradation of p53. Cancer Cell 2005; 8:75–87.
Wang GL, Shi X, Haefliger S, et al. Elimination of C/EBPα through the ubiquitin-proteasome system promotes the development of liver cancer in mice. J Clin Invest 2010; 120:2549–2562.
Richman RA, Claus TH, Pilkis SJ, Friedman DL . Hormonal stimulation of DNA synthesis in primary cultures of adult rat hepatocytes. Proc Natl Acad Sci USA 1976; 73:3589–3593.
Marker AJ, Galloway E, Palmer S, et al. Role of the adenylate cyclase, phosphoinositidase C and receptor tyrosyl kinase systems in the control of hepatocyte proliferation by hepatocyte growth factor. Biochem Pharmacol 1992; 44:1037–1043.
Levina E, Oren M, Ben-Ze'ev A . Downregulation of beta-catenin by p53 involves changes in the rate of beta-catenin phosphorylation and axin dynamics. Oncogene 2004; 23: 4444–4453.
Sadot E, Geiger B, Oren M, Ben-Ze'ev A . Down-regulation of beta-catenin by activated p53. Mol Cell Biol 2001; 21:6768–6781.
Man JH, Liang B, Gu YX, et al. Gankyrin plays an essential role in Ras-induced tumorigenesis through regulation of the RhoA/ROCK pathway in mammalian cells. J Clin Invest 2010; 120:2829–2841.
Yang JY, Zong CS, Xia W, et al. MDM2 promotes cell motility and invasiveness by regulating E-cadherin degradation. Mol Cell Biol 2006; 26:7269–7282.
Buendia MA . Genetics of hepatocellular carcinoma. Semin Cancer Biol 2000; 10:185–200.
Inagawa S, Itabashi M, Adachi S, et al. Expression and prognostic roles of beta-catenin in hepatocellular carcinoma: correlation with tumor progression and postoperative survival. Clin Cancer Res 2002; 8:450–456.
Kondo Y, Kanai Y, Sakamoto M, et al. Beta-catenin accumulation and mutation of exon 3 of the beta-catenin gene in hepatocellular carcinoma. Jpn J Cancer Res 1999; 90:1301–1309.
Yang W, Yan HX, Chen L, et al. Wnt/beta-catenin signaling contributes to activation of normal and tumorigenic liver progenitor cells. Cancer Res 2008; 68:4287–4295.
Datta SR, Dudek H, Tao X, et al. Akt phosphorylation of BAD couples survival signals to the cell-intrinsic death machinery. Cell 1997; 91:231–241.
Li H, Fu X, Chen Y, et al. Use of adenovirus-delivered siRNA to target oncoprotein p28GANK in hepatocellular carcinoma. Gastroenterology 2005; 128:2029–2041.
Eastman Q, Grosschedl R . Regulation of LEF-1/TCF transcription factors by Wnt and other signals. Curr Opin Cell Biol 1999; 11:233–240.
Umemura A, Itoh Y, Itoh K, et al. Association of gankyrin protein expression with early clinical stages and insulin-like growth factor-binding protein 5 expression in human hepatocellular carcinoma. Hepatology 2007; 47:493–502.
Levy L, Renard CA, Wei Y, Buendia MA . Genetic alterations and oncogenic pathways in hepatocellular carcinoma. Ann N Y Acad Sci 2002; 963:21–36.
Qin JM, Fu XY, Li SJ, et al. Gene and protein expressions of p28GANK in rat with liver regeneration. World J Gastroenterol 2003; 9:2523–2527.
Acknowledgements
We thank Dr Claudia Cosentino and Dr Jin Q Cheng for kindly providing some of the plasmids. We are also grateful to Dr David Blake and Dr Xiaoni Kong (School of Public Health, Johns Hopkins University, Baltimore, MD) for helpful discussion. Research was supported by grants from the Funds for Creative Research Groups of China (30921006), the State Key Project for Liver Cancer (2008ZX10002), the State Key Laboratory of Oncogenes and Related Genes (91-10-02, 91-10-03), the National Natural Science Foundation of China (81001075, 81071778).
Author information
Authors and Affiliations
Corresponding author
Additional information
( Supplementary information is linked to the online version of the paper on the Cell Research website.)
Rights and permissions
About this article
Cite this article
Dong, Lw., Yang, Gz., Pan, Yf. et al. The oncoprotein p28GANK establishes a positive feedback loop in β-catenin signaling. Cell Res 21, 1248–1261 (2011). https://doi.org/10.1038/cr.2011.103
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/cr.2011.103
Keywords
This article is cited by
-
The oncogene Gankyrin is expressed in testicular cancer and contributes to cisplatin sensitivity in embryonal carcinoma cells
BMC Cancer (2019)
-
Gankyrin as a potential therapeutic target for cancer
Investigational New Drugs (2017)
-
Gankyrin facilitates follicle-stimulating hormone-driven ovarian cancer cell proliferation through the PI3K/AKT/HIF-1α/cyclin D1 pathway
Oncogene (2016)
-
The co-expression of GPER and Gankyrin in ovarian endometriosis and its correlation with the rASRM stages
Archives of Gynecology and Obstetrics (2016)
-
Prognostic value of carbonic anhydrase VII expression in colorectal carcinoma
BMC Cancer (2015)