Abstract
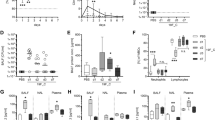
The 2009 flu pandemic involved the emergence of a new strain of a swine-origin H1N1 influenza virus (S-OIV H1N1) that infected almost every country in the world. Most infections resulted in respiratory illness and some severe cases resulted in acute lung injury. In this report, we are the first to describe a mouse model of S-OIV virus infection with acute lung injury and immune responses that reflect human clinical disease. The clinical efficacy of the antiviral oseltamivir (Tamiflu) administered in the early stages of S-OIV H1N1 infection was confirmed in the mouse model. Moreover, elevated levels of IL-17, Th-17 mediators and IL-17-responsive cytokines were found in serum samples of S-OIV-infected patients in Beijing. IL-17 deficiency or treatment with monoclonal antibodies against IL-17-ameliorated acute lung injury induced by the S-OIV H1N1 virus in mice. These results suggest that IL-17 plays an important role in S-OIV-induced acute lung injury and that monoclonal antibodies against IL-17 could be useful as a potential therapeutic remedy for future S-OIV H1N1 pandemics.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Hamilton K . The emergence of the pandemic A/H1N1 2009 virus and its characteristics. Bull Mem Acad R Med Belg 2009; 164:260–263.
Smith GJ, Vijaykrishna D, Bahl J, et al. Origins and evolutionary genomics of the 2009 swine-origin H1N1 influenza A epidemic. Nature 2009; 459:1122–1125.
Chowell G, Bertozzi SM, Colchero MA, et al. Severe respiratory disease concurrent with the circulation of H1N1 influenza. N Engl J Med 2009; 361:674–679.
Dominguez-Cherit G, Lapinsky SE, Macias AE, et al. Critically Ill patients with 2009 influenza A(H1N1) in Mexico. JAMA 2009; 302:1880–1887.
Louie JK, Acosta M, Winter K, et al. Factors associated with death or hospitalization due to pandemic 2009 influenza A(H1N1) infection in California. JAMA 2009; 302:1896–1902.
Bermejo-Martin JF, Ortiz de Lejarazu R, Pumarola T, et al. Th1 and Th17 hypercytokinemia as early host response signature in severe pandemic influenza. Crit Care 2009; 13:R201.
Hagau N, Slavcovici A, Gonganau DN, et al. Clinical aspects and cytokine response in severe H1N1 influenza A virus infection. Crit Care 2010; 14:R203.
To KK, Hung IF, Li IW, et al. Delayed clearance of viral load and marked cytokine activation in severe cases of pandemic H1N1 2009 influenza virus infection. Clin Infect Dis 2010; 50:850–859.
Maines TR, Jayaraman A, Belser JA, et al. Transmission and pathogenesis of swine-origin 2009 A(H1N1) influenza viruses in ferrets and mice. Science 2009; 325:484–487.
Munster VJ, de Wit E, van den Brand JM, et al. Pathogenesis and transmission of swine-origin 2009 A(H1N1) influenza virus in ferrets. Science 2009; 325:481–483.
Sun Y, Bi Y, Pu J, et al. Guinea pig model for evaluating the potential public health risk of swine and avian influenza viruses. PLoS One 2010; 5:e15537.
Meunier I, Pillet S, Simonsen JN, von Messling V . Influenza pathogenesis: lessons learned from animal studies with H5N1, H1N1 Spanish, and pandemic H1N1 2009 influenza. Crit Care Med 2010; 38(4 Suppl):e21–e29.
Chan KH, Zhang AJ, To KK, et al. Wild type and mutant 2009 pandemic influenza A (H1N1) viruses cause more severe disease and higher mortality in pregnant BALB/c mice. PLoS One 2010; 5:e13757.
Belser JA, Wadford DA, Pappas C, et al. Pathogenesis of pandemic influenza A (H1N1) and triple-reassortant swine influenza A (H1) viruses in mice. J Virol 2010; 84:4194–4203.
Mauad T, Hajjar LA, Callegari GD, et al. Lung pathology in fatal novel human influenza A (H1N1) infection. Am J Respir Crit Care Med 2010; 181:72–79.
Sun Y, Shi Y, Zhang W, et al. In silico characterization of the functional and structural modules of the hemagglutinin protein from the swine-origin influenza virus A (H1N1)-2009. Sci China Life Sci 2010; 53:633–642.
Gao GF, Sun Y . It is not just AIV: from avian to swine-origin influenza virus. Sci China Life Sci 2010; 53:151–153.
Quispe-Laime AM, Bracco JD, Barberio PA, et al. H1N1 influenza A virus-associated acute lung injury: response to combination oseltamivir and prolonged corticosteroid treatment. Intensive Care Med 2010; 36:33–41.
Hui DS, Lee N, Chan PK . Clinical management of pandemic 2009 influenza A(H1N1) infection. Chest 2010; 137:916–925.
Martin-Loeches I, Lisboa T, Rhodes A, et al. Use of early corticosteroid therapy on ICU admission in patients affected by severe pandemic (H1N1)v influenza A infection. Intensive Care Med 2011; 37:272–283.
Lee VJ, Yap J, Cook AR, et al. Oseltamivir ring prophylaxis for containment of 2009 H1N1 influenza outbreaks. N Engl J Med 2010; 362:2166–2174.
Goldstein E, Cowling BJ, O'Hagan JJ, et al. Oseltamivir for treatment and prevention of pandemic influenza A/H1N1 virus infection in households, Milwaukee, 2009. BMC Infect Dis 2010; 10:211.
Yu H, Liao Q, Yuan Y, et al. Effectiveness of oseltamivir on disease progression and viral RNA shedding in patients with mild pandemic 2009 influenza A H1N1: opportunistic retrospective study of medical charts in China. BMJ 2010; 341:c4779.
Steinman L . A brief history of T(H)17, the first major revision in the T(H)1/T(H)2 hypothesis of T cell-mediated tissue damage. Nat Med 2007; 13:139–145.
Cua DJ, Sherlock J, Chen Y, et al. Interleukin-23 rather than interleukin-12 is the critical cytokine for autoimmune inflammation of the brain. Nature 2003; 421:744–748.
Murphy CA, Langrish CL, Chen Y, et al. Divergent pro- and antiinflammatory roles for IL-23 and IL-12 in joint autoimmune inflammation. J Exp Med 2003; 198:1951–1957.
Park H, Li Z, Yang XO, et al. A distinct lineage of CD4 T cells regulates tissue inflammation by producing interleukin 17. Nat Immunol 2005; 6:1133–1141.
Hamada H, Garcia-Hernandez Mde L, Reome JB, et al. Tc17, a unique subset of CD8 T cells that can protect against lethal influenza challenge. J Immunol 2009; 182:3469–3481.
Crowe CR, Chen K, Pociask DA, et al. Critical role of IL-17RA in immunopathology of influenza infection. J Immunol 2009; 183:5301–5310.
Kudva A, Scheller EV, Robinson KM, et al. Influenza A inhibits Th17-mediated host defense against bacterial pneumonia in mice. J Immunol 2011; 186:1666–1674.
Reichert JM, Rosensweig CJ, Faden LB, Dewitz MC . Monoclonal antibody successes in the clinic. Nat Biotechnol 2005; 23:1073–1078.
Zhu J, Paul WE . Heterogeneity and plasticity of T helper cells. Cell Res 2010; 20:4–12.
Xu S, Cao X . Interleukin-17 and its expanding biological functions. Cell Mol Immunol 2010; 7:164–174.
Chang SH, Dong C . Signaling of interleukin-17 family cytokines in immunity and inflammation. Cell Signal 2011; 23:1069–1075.
Xu G, Zhang L, Wang DY, et al. Opposing roles of IL-17A and IL-25 in the regulation of TSLP production in human nasal epithelial cells. Allergy 2010; 65:581–589.
Miossec P, Korn T, Kuchroo VK . Interleukin-17 and type 17 helper T cells. N Engl J Med 2009; 361:888–898.
Hueber W, Patel DD, Dryja T, et al. Effects of AIN457, a fully human antibody to interleukin-17A, on psoriasis, rheumatoid arthritis, and uveitis. Sci Transl Med 2010; 2:52ra72.
Acknowledgements
We thank Zongsheng Han, Yunli Ling and Ran Zhang for technical support. This work was supported by the National Natural Science Foundation of China (30625013 and 30623009), the Ministry of Science and Technology of China (2009CB522102 and 2009CB522105) and the Ministry of Health (2009ZX10004-308). Dr Zhinan Yin was supported by the National Outstanding Young Scientist Award of National Natural Science Foundation of China (30725015).
Author information
Authors and Affiliations
Corresponding authors
Additional information
(Supplementary information is linked to the online version of the paper on the Cell Research website.)
Supplementary information
Supplementary information, Table S1
Basic information of patients recruited. (PDF 867 kb)
Supplementary information, Figure S1
Flow chart of S-OIV-infected patient recruitment and sample collection in the hospitals in Beijing. (PDF 495 kb)
Supplementary information, Figure S2
Acute lung injury induced by S-OIV H1N1 in mice is not improved by administration of monoclonal antibodies against TNF-alpha. (PDF 375 kb)
Supplementary information, Figure S3
Monoclonal antibodies against IL-17 do not alter the replication of S-OIV H1N1 in mice. (PDF 510 kb)
Supplementary information, Data S1
Materials and Methods (PDF 127 kb)
Rights and permissions
About this article
Cite this article
Li, C., Yang, P., Sun, Y. et al. IL-17 response mediates acute lung injury induced by the 2009 Pandemic Influenza A (H1N1) Virus. Cell Res 22, 528–538 (2012). https://doi.org/10.1038/cr.2011.165
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/cr.2011.165
Keywords
This article is cited by
-
An IL-17-EGFR-TRAF4 axis contributes to the alleviation of lung inflammation in severe influenza
Communications Biology (2023)
-
IL-17 and IL-17-producing cells in protection versus pathology
Nature Reviews Immunology (2023)
-
Acinetobacter baumannii reinforces the pathogenesis by promoting IL-17 production in a mouse pneumonia model
Medical Microbiology and Immunology (2023)
-
Galangin for COVID-19 and Mucormycosis co-infection: a potential therapeutic strategy of targeting critical host signal pathways triggered by SARS-CoV-2 and Mucormycosis
Network Modeling Analysis in Health Informatics and Bioinformatics (2023)
-
Targeting the IL-22/IL-22BP axis enhances tight junctions and reduces inflammation during influenza infection
Mucosal Immunology (2020)