Abstract
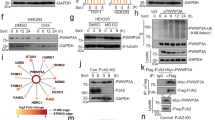
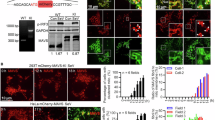
Recognition of viral RNA structures by the cytosolic sensor retinoic acid-inducible gene-I (RIG-I) results in the activation of signaling cascades that culminate with the generation of the type I interferon (IFN) antiviral response. Onset of antiviral and inflammatory responses to viral pathogens necessitates the regulated spatiotemporal recruitment of signaling adapters, kinases and transcriptional proteins to the mitochondrial antiviral signaling protein (MAVS). We previously demonstrated that the serine/threonine kinase IKKε is recruited to the C-terminal region of MAVS following Sendai or vesicular stomatitis virus (VSV) infection, mediated by Lys63-linked polyubiquitination of MAVS at Lys500, resulting in inhibition of downstream IFN signaling (Paz et al, Mol Cell Biol, 2009). In this study, we demonstrate that C-terminus of MAVS harbors a novel TRAF3-binding site in the aa450-468 region of MAVS. A consensus TRAF-interacting motif (TIM), 455-PEENEY-460, within this site is required for TRAF3 binding and activation of IFN antiviral response genes, whereas mutation of the TIM eliminates TRAF3 binding and the downstream IFN response. Reconstitution of MAVS−/− mouse embryo fibroblasts with a construct expressing a TIM-mutated version of MAVS failed to restore the antiviral response or block VSV replication, whereas wild-type MAVS reconstituted antiviral inhibition of VSV replication. Furthermore, recruitment of IKKε to an adjacent C-terminal site (aa 468–540) in MAVS via Lys500 ubiquitination decreased TRAF3 binding and protein stability, thus contributing to IKKε-mediated shutdown of the IFN response. This study demonstrates that MAVS harbors a functional C-terminal TRAF3-binding site that participates in positive and negative regulation of the IFN antiviral response.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Samuel CE . Antiviral actions of interferons. Clin Microbiol Rev 2001; 14:778–809.
Yoneyama M, Fujita T . Recognition of viral nucleic acids in innate immunity. Rev Med Virol 2010; 20:4–22.
Akira S, Uematsu S, Takeuchi O . Pathogen recognition and innate immunity. Cell 2006; 124:783–801.
Uematsu S, Akira S . Toll-like receptors and innate immunity. J Mol Med 2006; 84:712–725.
Schindler C, Levy DE, Decker T . JAK-STAT signaling: from interferons to cytokines. J Biol Chem 2007; 282:20059–20063.
Yoneyama M, Kikuchi M, Natsukawa T, et al. The RNA helicase RIG-I has an essential function in double-stranded RNA-induced innate antiviral responses. Nat Immunol 2004; 5:730–737.
Yoneyama M, Fujita T . Function of RIG-I-like receptors in antiviral innate immunity. J Biol Chem 2007; 282:15315–15318.
Wilkins C . Gale M Jr . Recognition of viruses by cytoplasmic sensors. Curr Opin Immunol 2010; 22:41–47.
Komuro A, Horvath CM . RNA- and virus-independent inhibition of antiviral signaling by RNA helicase LGP2. J Virol 2006; 80:12332–12342.
Kawai T, Takahashi K, Sato S, et al. IPS-1, an adaptor triggering RIG-I- and Mda5-mediated type I interferon induction. Nat Immunol 2005; 6:981–988.
Meylan E, Curran J, Hofmann K, et al. Cardif is an adaptor protein in the RIG-I antiviral pathway and is targeted by hepatitis C virus. Nature 2005; 437:1167–1172.
Seth RB, Sun L, Ea CK, Chen ZJ . Identification and characterization of MAVS, a mitochondrial antiviral signaling protein that activates NF-kappaB and IRF 3. Cell 2005; 122:669–682.
Xu LG, Wang YY, Han KJ, Li LY, Zhai Z, Shu HB . VISA is an adapter protein required for virus-triggered IFN-beta signaling. Mol Cell 2005; 19:727–740.
Lin R, Lacoste J, Nakhaei P, et al. Dissociation of a MAVS/IPS-1/VISA/Cardif-IKKepsilon molecular complex from the mitochondrial outer membrane by hepatitis C virus NS3-4A proteolytic cleavage. J Virol 2006; 80:6072–6083.
Paz S, Vilasco M, Arguello M, et al. Ubiquitin-regulated recruitment of IkappaB kinase epsilon to the MAVS interferon signaling adapter. Mol Cell Biol 2009; 29:3401–3412.
Baril M, Racine ME, Penin F, Lamarre D . MAVS dimer is a crucial signaling component of innate immunity and the target of hepatitis C virus NS3/4A protease. J Virol 2009; 83:1299–1311.
Tang ED, Wang CY . MAVS self-association mediates antiviral innate immune signaling. J Virol 2009; 83:3420–3428.
Saha SK, Pietras EM, He JQ, et al. Regulation of antiviral responses by a direct and specific interaction between TRAF3 and Cardif. EMBO J 2006; 25:3257–3263.
Lin R, Paz S, Hiscott J . Tom70 imports antiviral immunity to the mitochondria. Cell Res 2010; 20:971–973.
Michallet MC, Meylan E, Ermolaeva MA, et al. TRADD protein is an essential component of the RIG-like helicase antiviral pathway. Immunity 2008; 28:651–661.
Balachandran S, Thomas E, Barber GN . A FADD-dependent innate immune mechanism in mammalian cells. Nature 2004; 432:401–405.
Tang ED, Wang CY . TRAF5 is a downstream target of MAVS in antiviral innate immune signaling. PLoS One 2010; 5:e9172.
Ishikawa H, Barber GN . STING is an endoplasmic reticulum adaptor that facilitates innate immune signalling. Nature 2008; 455:674–678.
Zhong B, Yang Y, Li S, et al. The adaptor protein MITA links virus-sensing receptors to IRF3 transcription factor activation. Immunity 2008; 29:538–550.
Sun W, Li Y, Chen L, et al. ERIS, an endoplasmic reticulum IFN stimulator, activates innate immune signaling through dimerization. Proc Natl Acad Sci USA 2009; 106:8653–8658.
Nakhaei P, Genin P, Civas A, Hiscott J . RIG-I-like receptors: sensing and responding to RNA virus infection. Semin Immunol 2009; 21:215–222.
Moore CB, Bergstralh DT, Duncan JA, et al. NLRX1 is a regulator of mitochondrial antiviral immunity. Nature 2008; 451:573–577.
You F, Sun H, Zhou X, et al. PCBP2 mediates degradation of the adaptor MAVS via the HECT ubiquitin ligase AIP4. Nat Immunol 2009; 10:1300–1308.
Vitour D, Dabo S . Ahmadi Pour M, et al. Polo-like kinase 1 (PLK1) regulates interferon (IFN) induction by MAVS. J Biol Chem 2009; 284:21797–21809.
Mankouri J, Fragkoudis R . Richards KH, et al. Optineurin negatively regulates the induction of IFNbeta in response to RNA virus infection. PLoS Pathog 2010; 6:e1000778.
Kayagaki N, Phung Q, Chan S, et al. DUBA: a deubiquitinase that regulates type I interferon production. Science 2007; 318:1628–1632.
Lin R, Yang L, Nakhaei P, et al. Negative regulation of the retinoic acid-inducible gene I-induced antiviral state by the ubiquitin-editing protein A20. J Biol Chem 2006; 281:2095–2103.
He JQ, Oganesyan G, Saha SK, Zarnegar B, Cheng G . TRAF3 and its biological function. Adv Exp Med Biol 2007; 597:48–59.
Dempsey PW, Doyle SE, He JQ, Cheng G . The signaling adaptors and pathways activated by TNF superfamily. Cytokine Growth Factor Rev 2003; 14:193–209.
Oganesyan G, Saha SK, Guo B, et al. Critical role of TRAF3 in the Toll-like receptor-dependent and -independent antiviral response. Nature 2006; 439:208–211.
Karin M . How NF-kappaB is activated: the role of the IkappaB kinase (IKK) complex. Oncogene 1999; 18:6867–6874.
Karin M, Ben-Neriah Y . Phosphorylation meets ubiquitination: The control of NF-κB activity. Ann Rev Immunol 2000; 18:621–663.
Perkins ND . Post-translational modifications regulating the activity and function of the nuclear factor kappa B pathway. Oncogene 2006; 25:6717–6730.
Perkins ND . Integrating cell-signalling pathways with NF-kappaB and IKK function. Nat Rev Mol Cell Biol 2007; 8:49–62.
Sharma S, tenOever BR, Grandvaux N, Zhou GP, Lin R, Hiscott J . Triggering the interferon antiviral response through an IKK-related pathway. Science 2003; 300:1148–1151.
Paz S, Sun Q, Nakhaei P, et al. Induction of IRF-3 and IRF-7 phosphorylation following activation of the RIG-I pathway. Cell Mol Biol (Noisy-le-grand) 2006; 52:17–28.
Fitzgerald KA, McWhirter SM, Faia KL, et al. IKKepsilon and TBK1 are essential components of the IRF3 signaling pathway. Nat Immunol 2003; 4:491–496.
Panne D, McWhirter SM, Maniatis T, Harrison SC . Interferon regulatory factor 3 is regulated by a dual phosphorylation-dependent switch. J Biol Chem 2007; 282:22816–22822.
Saitoh T, Yamamoto M, Miyagishi M, et al. A20 is a negative regulator of IFN regulatory factor 3 signaling. J Immunol 2005; 174:1507–1512.
Wang YY, Li L, Han KJ, Zhai Z, Shu HB . A20 is a potent inhibitor of TLR3- and Sendai virus-induced activation of NF-kappaB and ISRE and IFN-beta promoter. FEBS Lett 2004; 576:86–90.
Xu L, Xiao N, Liu F, Ren H, Gu J . Inhibition of RIG-I and MDA5-dependent antiviral response by gC1qR at mitochondria. Proc Natl Acad Sci USA 2009; 106:1530–1535.
Sanada T, Takaesu G, Mashima R, Yoshida R, Kobayashi T, Yoshimura A . FLN29 deficiency reveals its negative regulatory role in the Toll-like receptor (TLR) and retinoic acid-inducible gene I (RIG-I)-like helicase signaling pathway. J Biol Chem 2008; 283:33858–33864.
Zhang M, Wu X . Lee AJ, et al. Regulation of IkappaB kinase-related kinases and antiviral responses by tumor suppressor CYLD. J Biol Chem 2008; 283:18621–18626.
Friedman CS, O'Donnell MA, Legarda-Addison D, et al. The tumour suppressor CYLD is a negative regulator of RIG-I-mediated antiviral response. EMBO Rep 2008; 9:930–936.
Jia Y, Song T, Wei C, et al. Negative regulation of MAVS-mediated innate immune response by PSMA7. J Immunol 2009; 183:4241–4248.
Hou J, Wang P, Lin L, et al. MicroRNA-146a feedback inhibits RIG-I-dependent Type I IFN production in macrophages by targeting TRAF6, IRAK1, and IRAK2. J Immunol 2009; 183:2150–2158.
Cui J, Zhu L, Xia X, et al. NLRC5 negatively regulates the NF-kappaB and type I interferon signaling pathways. Cell 2010; 141:483–496.
Saito T, Hirai R, Loo YM, et al. Regulation of innate antiviral defenses through a shared repressor domain in RIG-I and LGP2. Proc Natl Acad Sci USA 2007; 104:582–587.
Kim MJ, Hwang SY, Imaizumi T, Yoo JY . Negative feedback regulation of RIG-I-mediated antiviral signaling by interferon-induced ISG15 conjugation. J Virol 2008; 82:1474–1483.
Arimoto K, Takahashi H, Hishiki T, Konishi H, Fujita T, Shimotohno K . Negative regulation of the RIG-I signaling by the ubiquitin ligase RNF125. Proc Natl Acad Sci USA 2007; 104:7500–7505.
Saitoh T, Tun-Kyi A, Ryo A, et al. Negative regulation of interferon-regulatory factor 3-dependent innate antiviral response by the prolyl isomerase Pin1. Nat Immunol 2006; 7:598–605.
Huang J, Liu T, Xu LG, Chen D, Zhai Z, Shu HB . SIKE is an IKK epsilon/TBK1-associated suppressor of TLR3- and virus-triggered IRF-3 activation pathways. EMBO J 2005; 24:4018–4028.
Liu XY, Wei B, Shi HX, Shan YF, Wang C . Tom70 mediates activation of interferon regulatory factor 3 on mitochondria. Cell Res 2010; 20:994–1011.
Nakhaei P, Mesplede T . Solis M, et al. The E3 ubiquitin ligase Triad3A negatively regulates the RIG-I/MAVS signaling pathway by targeting TRAF3 for degradation. PLoS Pathog 2009; 5:e1000650.
Tseng PH, Matsuzawa A, Zhang W, Mino T, Vignali DA, Karin M . Different modes of ubiquitination of the adaptor TRAF3 selectively activate the expression of type I interferons and proinflammatory cytokines. Nat Immunol 2010; 11:70–75.
Yoshida R, Takaesu G . Yoshida H, et al. TRAF6 and MEKK1 play a pivotal role in the RIG-I-like helicase antiviral pathway. J Biol Chem 2008; 283:36211–36220.
Sun Q, Sun L, Liu HH, et al. The specific and essential role of MAVS in antiviral innate immune responses. Immunity 2006; 24:633–642.
Stojdl DF, Lichty B, Knowles S, et al. Exploiting tumor-specific defects in the interferon pathway with a previously unknown oncolytic virus. Nat Med 2000; 6:821–825.
Stojdl DF, Lichty BD, tenOever BR, et al. VSV strains with defects in their ability to shutdown innate immunity are potent systemic anti-cancer agents. Cancer Cell 2003; 4:263–275.
Servant MJ, Grandvaux N, tenOever BR, Duguay D, Lin R, Hiscott J . Identification of the minimal phosphoacceptor site required for in vivo activation of interferon regulatory factor 3 in response to virus and double-stranded RNA. J Biol Chem 2003; 278:9441–9447.
Lin R, Heylbroeck C, Pitha PM, Hiscott J . Virus-dependent phosphorylation of the IRF-3 transcription factor regulates nuclear translocation, transactivation potential, and proteasome-mediated degradation. Mol Cell Biol 1998; 18:2986–2996.
Bibeau-Poirier A, Gravel SP, Clement JF, et al. Involvement of the IkappaB kinase (IKK)-related kinases tank-binding kinase 1/IKKi and cullin-based ubiquitin ligases in IFN regulatory factor-3 degradation. J Immunol 2006; 177:5059–5067.
Gack MU, Shin YC, Joo CH, et al. TRIM25 RING-finger E3 ubiquitin ligase is essential for RIG-I-mediated antiviral activity. Nature 2007; 446:916–920.
Hiscott J . Triggering the innate antiviral response through IRF-3 activation. J Biol Chem 2007; 282:15325–15329.
Dixit E, Boulant S, Zhang Y, et al. Peroxisomes are signaling platforms for antiviral innate immunity. Cell 2010; 141:668–681.
Hoepfner D, Schildknegt D, Braakman I, Philippsen P, Tabak HF . Contribution of the endoplasmic reticulum to peroxisome formation. Cell 2005; 122:85–95.
Zeng W, Sun L, Jiang X, et al. Reconstitution of the RIG-I pathway reveals a signaling role of unanchored polyubiquitin chains in innate immunity. Cell 2010; 141:315–330.
Acknowledgements
This work was supported by grants from the Canadian Institutes for Health Research, Canadian Cancer Society and the Canadian Foundation for AIDS Research, awarded to JH and grants from L'Agence Nationale de la Recherche contre le SIDA (ANRS), awarded to EM. SP is the recipient of an FRSQ Bourse de Troisième Cycle (Doctorat) and MV was the recipient of a FRM Bourse de Fin de Thèse.
Author information
Authors and Affiliations
Corresponding author
Additional information
( Supplementary information is linked to the online version of the paper on the Cell Research website.)
Supplementary information
Supplementary information, Figure S1
N-terminal binding of TRAF3 to MAVS is not required for IFN activation. (PDF 110 kb)
Rights and permissions
About this article
Cite this article
Paz, S., Vilasco, M., Werden, S. et al. A functional C-terminal TRAF3-binding site in MAVS participates in positive and negative regulation of the IFN antiviral response. Cell Res 21, 895–910 (2011). https://doi.org/10.1038/cr.2011.2
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/cr.2011.2
Keywords
This article is cited by
-
Early innate immune response triggered by the human respiratory syncytial virus and its regulation by ubiquitination/deubiquitination processes
Journal of Biomedical Science (2022)
-
MST4 negatively regulates type I interferons production via targeting MAVS-mediated pathway
Cell Communication and Signaling (2022)
-
Influenza a virus NS1 resembles a TRAF3-interacting motif to target the RNA sensing-TRAF3-type I IFN axis and impair antiviral innate immunity
Journal of Biomedical Science (2021)
-
RNF115 plays dual roles in innate antiviral responses by catalyzing distinct ubiquitination of MAVS and MITA
Nature Communications (2020)
-
TRAF3IP3 negatively regulates cytosolic RNA induced anti-viral signaling by promoting TBK1 K48 ubiquitination
Nature Communications (2020)