Abstract
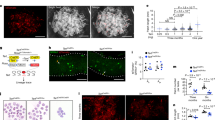
Rejuvenation of telomeres with various lengths has been found in induced pluripotent stem cells (iPSCs). Mechanisms of telomere length regulation during induction and proliferation of iPSCs remain elusive. We show that telomere dynamics are variable in mouse iPSCs during reprogramming and passage, and suggest that these differences likely result from multiple potential factors, including the telomerase machinery, telomerase-independent mechanisms and clonal influences including reexpression of exogenous reprogramming factors. Using a genetic model of telomerase-deficient (Terc−/− and Terc+/−) cells for derivation and passages of iPSCs, we found that telomerase plays a critical role in reprogramming and self-renewal of iPSCs. Further, telomerase maintenance of telomeres is necessary for induction of true pluripotency while the alternative pathway of elongation and maintenance by recombination is also required, but not sufficient. Together, several aspects of telomere biology may account for the variable telomere dynamics in iPSCs. Notably, the mechanisms employed to maintain telomeres during iPSC reprogramming are very similar to those of embryonic stem cells. These findings may also relate to the cloning field where these mechanisms could be responsible for telomere heterogeneity after nuclear reprogramming by somatic cell nuclear transfer.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Takahashi K, Yamanaka S . Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 2006; 126:663–676.
Wernig M, Meissner A . Foreman R, et al. In vitro reprogramming of fibroblasts into a pluripotent ES-cell-like state. Nature 2007; 448:318–324.
Okita K, Ichisaka T, Yamanaka S . Generation of germline-competent induced pluripotent stem cells. Nature 2007; 448:313–317.
Jaenisch R, Young R . Stem cells, the molecular circuitry of pluripotency and nuclear reprogramming. Cell 2008; 132:567–582.
Zhao XY, Li W, Lv Z, et al. iPS cells produce viable mice through tetraploid complementation. Nature 2009; 461:86–90.
Boland MJ, Hazen JL, Nazor KL, et al. Adult mice generated from induced pluripotent stem cells. Nature 2009; 461:91–94.
Blackburn EH . Switching and signaling at the telomere. Cell 2001; 106:661–673.
Palm W, de Lange T . How shelterin protects mammalian telomeres. Annu Rev Genet 2008; 42:301–334.
Wang Y, Erdmann N . Giannone RJ, et al. An increase in telomere sister chromatid exchange in murine embryonic stem cells possessing critically shortened telomeres. Proc Natl Acad Sci USA 2005; 102:10256–10260.
Hiyama E, Hiyama K . Telomere and telomerase in stem cells. Br J Cancer 2007; 96:1020–1024.
Wright WE, Piatyszek MA, Rainey WE, Byrd W, Shay JW . Telomerase activity in human germline and embryonic tissues and cells. Dev Genet 1996; 18:173–179.
Forsyth NR, Wright WE, Shay JW . Telomerase and differentiation in multicellular organisms: turn it off, turn it on, and turn it off again. Differentiation 2002; 69:188–197.
Wright WE, Shay JW . Historical claims and current interpretations of replicative aging. Nat Biotechnol 2002; 20:682–688.
Blackburn EH . Telomere states and cell fates. Nature 2000; 408:53–56.
Gomes NM, Shay JW, Wright WE . Telomere biology in Metazoa. FEBS Lett 2010; 584:3741–3751.
Blasco MA . The epigenetic regulation of mammalian telomeres. Nat Rev Genet 2007; 8:299–309.
Benetti R, Gonzalo S, Jaco I, et al. Suv4-20h deficiency results in telomere elongation and derepression of telomere recombination. J Cell Biol 2007; 178:925–936.
Gonzalo S, Jaco I, Fraga MF, et al. DNA methyltransferases control telomere length and telomere recombination in mammalian cells. Nat Cell Biol 2006; 8:416–424.
Zalzman M, Falco G . Sharova LV, et al. Zscan4 regulates telomere elongation and genomic stability in ES cells. Nature 2010; 464:858–863.
Marion RM, Strati K, Li H, et al. Telomeres acquire embryonic stem cell characteristics in induced pluripotent stem cells. Cell Stem Cell 2009; 4:141–154.
Agarwal S, Loh YH, McLoughlin EM, et al. Telomere elongation in induced pluripotent stem cells from dyskeratosis congenita patients. Nature 2010; 464:292–296.
Vaziri H, Chapman KB, Guigova A, et al. Spontaneous reversal of the developmental aging of normal human cells following transcriptional reprogramming. Regen Med 2010; 5:345–363.
Suhr ST, Chang EA, Rodriguez RM, et al. Telomere dynamics in human cells reprogrammed to pluripotency. PLoS One 2009; 4:e8124.
Mathew R, Jia W, Sharma A, et al. Robust activation of the human but not mouse telomerase gene during the induction of pluripotency. FASEB J 2010; 24:2702–2715.
Takahashi K, Tanabe K, Ohnuki M, et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell 2007; 131:861–872.
Stadtfeld M, Maherali N, Breault DT, Hochedlinger K . Defining molecular cornerstones during fibroblast to iPS cell reprogramming in mouse. Cell Stem Cell 2008; 2:230–240.
Batista LF, Pech MF, Zhong FL, et al. Telomere shortening and loss of self-renewal in dyskeratosis congenita induced pluripotent stem cells. Nature 2011; 474:399–402.
Agarwal S, Daley GQ . Telomere dynamics in dyskeratosis congenita: the long and the short of iPS. Cell Res 2011; 21:1157–1160.
Huang J, Wang F, Okuka M, et al. Association of telomere length with authentic pluripotency of ES/iPS cells. Cell Res 2011; 21:779–792.
Herrera E, Samper E . Martin-Caballero J, et al. Disease states associated with telomerase deficiency appear earlier in mice with short telomeres. EMBO J 1999; 18:2950–2960.
Bailey SM, Brenneman MA, Goodwin EH . Frequent recombination in telomeric DNA may extend the proliferative life of telomerase-negative cells. Nucleic Acids Res 2004; 32:3743–3751.
Londono-Vallejo JA, Der-Sarkissian H, Cazes L, Bacchetti S, Reddel RR . Alternative lengthening of telomeres is characterized by high rates of telomeric exchange. Cancer Res 2004; 64:2324–2327.
Liu L, Bailey SM, Okuka M, et al. Telomere lengthening early in development. Nat Cell Biol 2007; 9:1436–1441.
Morrish TA, Greider CW . Short telomeres initiate telomere recombination in primary and tumor cells. PLoS Genet 2009; 5:e 1000357.
Wong CW, Hou PS, Tseng SF, et al. Kruppel-like transcription factor 4 contributes to maintenance of telomerase activity in stem cells. Stem Cells 2010; 28:1510–1517.
Koche RP, Smith ZD, Adli M, et al. Reprogramming factor expression initiates widespread targeted chromatin remodeling. Cell Stem Cell 2011; 8:96–105.
Liu Y, Kha H, Ungrin M, Robinson MO, Harrington L . Preferential maintenance of critically short telomeres in mammalian cells heterozygous for mTert. Proc Natl Acad Sci USA 2002; 99:3597–3602.
Hanna J, Saha K, Pando B, et al. Direct cell reprogramming is a stochastic process amenable to acceleration. Nature 2009; 462:595–601.
Polo JM, Liu S, Figueroa ME, et al. Cell type of origin influences the molecular and functional properties of mouse induced pluripotent stem cells. Nat Biotechnol 2010; 28:848–855.
Chin MH, Pellegrini M, Plath K, Lowry WE . Molecular analyses of human induced pluripotent stem cells and embryonic stem cells. Cell Stem Cell 2010; 7:263–269.
Kim K, Doi A, Wen B, et al. Epigenetic memory in induced pluripotent stem cells. Nature 2010; 467:285–290.
Guo G, Yang J, Nichols J, et al. Klf4 reverts developmentally programmed restriction of ground state pluripotency. Development 2009; 136:1063–1069.
Wu KJ, Grandori C . Amacker M, et al. Direct activation of TERT transcription by c-MYC. Nat Genet 1999; 21:220–224.
Flores I, Evan G, Blasco MA . Genetic analysis of myc and telomerase interactions in vivo. Mol Cell Biol 2006; 26:6130–6138.
Mikkelsen TS, Hanna J, Zhang X, et al. Dissecting direct reprogramming through integrative genomic analysis. Nature 2008; 454:49–55.
Hagelstrom RT, Blagoev KB, Niedernhofer LJ, Goodwin EH, Bailey SM . Hyper telomere recombination accelerates replicative senescence and may promote premature aging. Proc Natl Acad Sci USA 2010; 107:15768–15773.
Meissner A, Wernig M, Jaenisch R . Direct reprogramming of genetically unmodified fibroblasts into pluripotent stem cells. Nat Biotechnol 2007; 25:1177–1181.
Huang J, Deng K, Wu H, et al. Efficient production of mice from embryonic stem cells injected into four- or eight-cell embryos by piezo micromanipulation. Stem Cells 2008; 26:1883–1890.
Huangfu D, Maehr R, Guo W, et al. Induction of pluripotent stem cells by defined factors is greatly improved by small-molecule compounds. Nat Biotechnol 2008; 26:795–797.
Acknowledgements
We thank Dr Susan Bailey's lab for help and advice on CO-FISH experiments. This work was supported by MOST National Major Basic Research Program (2009CB941000), the Ministry of Agriculture of China Transgenic Special Program (2009ZX08006-010B) and NIH 2 R01 DK054369 (XP and HZ).
Author information
Authors and Affiliations
Corresponding author
Additional information
( Supplementary information is linked to the online version of the paper on the Cell Research website.)
Supplementary information
Supplementary information, Figure S1
(A) Expression of Tert and Terc by qPCR analysis of N33 (ESCs), MEF(mouse embryonic fibroblasts), WTfb (Tail-tip fibroblasts (TTF) from adult wild-type mice at the age of 8 weeks, and Kofb (Tail-tips fibroblasts from adult telomerase Terc deficient mice). (PDF 53 kb)
Supplementary information, Figure S2
Dynamics of telomerase and telomeres during induction of iPSCs from telomerase Terc knockout (KO, Terc−/−) TTF. (PDF 91 kb)
Supplementary information, Figure S3
Telomerase activity and maintenance of telomeres during passages of telomerase haplo-insufficient heterozygous (Het., Terc+/−) iPSCs. (PDF 71 kb)
Supplementary information, Figure S4
Expression of endogenous and exogenous genes of iPSCs determined by real-time PCR analysis. (PDF 90 kb)
Supplementary information, Figure S5
Relative amount of exogenous transcript as a proportion of total transcripts in iPSCs determined by real-time PCR analysis. (PDF 87 kb)
Supplementary information, Figure S6
Differentiation tests in vitro of Terc-deficient iPSCs at early and late passages. (PDF 150 kb)
Supplementary information, Figure S7
Association of telomere shortening and dysfunction with reduced developmental pluripotency of iPSCs. (PDF 129 kb)
Supplementary information, Table S1
Karyotyping of iPSC lines (PDF 6 kb)
Supplementary information, Table S2
Telomere loss and chromosome fusion of iPSCs at early passage (P6) (PDF 6 kb)
Supplementary information, Table S3
Chimeras of iPSCs generated by injection into 8-cell albino ICR embryos (PDF 6 kb)
Supplementary information, Table S4
Comparison of telomere length and efficiency (%) of chimeras (PDF 6 kb)
Supplementary information, Table S5
Primers for PCR analysis (PDF 45 kb)
Supplementary information, Table S6
Ratio of contribution of iPSCs in the chimeras by microsatellite analysis (PDF 31 kb)
Rights and permissions
About this article
Cite this article
Wang, F., Yin, Y., Ye, X. et al. Molecular insights into the heterogeneity of telomere reprogramming in induced pluripotent stem cells. Cell Res 22, 757–768 (2012). https://doi.org/10.1038/cr.2011.201
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/cr.2011.201
Keywords
This article is cited by
-
A ride through the epigenetic landscape: aging reversal by reprogramming
GeroScience (2021)
-
The role of telomere-binding modulators in pluripotent stem cells
Protein & Cell (2020)
-
Directed reprogramming of comprehensively characterized dental pulp stem cells extracted from natal tooth
Scientific Reports (2018)
-
Feeders facilitate telomere maintenance and chromosomal stability of embryonic stem cells
Nature Communications (2018)
-
Telomere heterogeneity linked to metabolism and pluripotency state revealed by simultaneous analysis of telomere length and RNA-seq in the same human embryonic stem cell
BMC Biology (2017)