Abstract
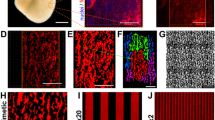
The recent breakthrough in the generation of rat embryonic stem cells (rESCs) opens the door to application of gene targeting to create models for the study of human diseases. In addition, the in vitro differentiation system from rESCs into derivatives of three germ layers will serve as a powerful tool and resource for the investigation of mammalian development, cell function, tissue repair, and drug discovery. However, these uses have been limited by the difficulty of in vitro differentiation. The aims of this study were to establish an in vitro differentiation system from rESCs and to investigate whether rESCs are capable of forming terminal-differentiated cardiomyocytes. Using newly established rESCs, we found that embryoid body (EB)-based method used in mouse ESC (mESC) differentiation failed to work for the serum-free cultivated rESCs. We then developed a protocol by combination of three chemical inhibitors and feeder-conditioned medium. Under this condition, rESCs formed EBs, propagated and differentiated into three embryonic germ layers. Moreover, rESC-formed EBs could differentiate into spontaneously beating cardiomyocytes after plating. Analyses of molecular, structural, and functional properties revealed that rESC-derived cardiomyocytes were similar to those derived from fetal rat hearts and mESCs. In conclusion, we successfully developed an in vitro differentiation system for rESCs through which functional myocytes were generated and displayed phenotypes of rat fetal cardiomyocytes. This unique cellular system will provide a new approach to study the early development and cardiac function, and serve as an important tool in pharmacological testing and cell therapy.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Aitman TJ, Critser JK, Cuppen E, et al. Progress and prospects in rat genetics: a community view. Nat Genet 2008; 40:516–522.
Jacob HJ, Kwitek AE . Rat genetics: attaching physiology and pharmacology to the genome. Nat Rev Genet 2002; 3:33–42.
Jacob HJ . Functional genomics and rat models. Genome Res 1999; 9:1013–1016.
Doggrell SA, Brown L . Rat models of hypertension, cardiac hypertrophy and failure. Cardiovasc Res 1998; 39:89–105.
Bader M . Rat models of cardiovascular diseases. Methods Mol Biol 2010; 597:403–414.
Buehr M, Meek S, Blair K, et al. Capture of authentic embryonic stem cells from rat blastocysts. Cell 2008; 135:1287–1298.
Li P, Tong C, Mehrian-Shai R, et al. Germline competent embryonic stem cells derived from rat blastocysts. Cell 2008; 135:1299–1310.
Hirabayashi M, Kato M, Kobayashi T, et al. Establishment of rat embryonic stem cell lines that can participate in germline chimerae at high efficiency. Mol Reprod Dev 2010; 77:94.
Kawamata M, Ochiya T . Establishment of embryonic stem cells from rat blastocysts. Methods Mol Biol 2010; 597:169–177.
Demers SP, Smith LC . Derivation, culture, and in vivo developmental capacity of embryonic cell lines from rat blastocysts. Methods Mol Biol 2010; 597:179–188.
Zhao X, Lv Z, Liu L, et al. Derivation of embryonic stem cells from Brown Norway rats blastocysts. J Genet Genomics 2010; 37:467–473.
Kawamata M, Ochiya T . Generation of genetically modified rats from embryonic stem cells. Proc Natl Acad Sci USA 2010; 107:14223–14228.
Dolgin E . The knockout rat pack. Nat Med 2010; 16:254–257.
Tong C, Li P, Wu NL, Yan Y, Ying QL . Production of p53 gene knockout rats by homologous recombination in embryonic stem cells. Nature 2010; 467:211–213.
Keller GM . In vitro differentiation of embryonic stem cells. Curr Opin Cell Biol 1995; 7:862–869.
Smith AG . Embryo-derived stem cells: of mice and men. Annu Rev Cell Dev Biol 2001; 17:435–462.
Keller G . Embryonic stem cell differentiation: emergence of a new era in biology and medicine. Genes Dev 2005; 19:1129–1155.
Wobus AM, Guan K, Yang HT, Boheler KR . Embryonic stem cells as a model to study cardiac, skeletal muscle, and vascular smooth muscle cell differentiation. Methods Mol Biol 2002; 185:127–156.
Boheler KR, Czyz J, Tweedie D, et al. Differentiation of pluripotent embryonic stem cells into cardiomyocytes. Circ Res 2002; 91:189–201.
Winkler J, Hescheler J, Sachinidis A . Embryonic stem cells for basic research and potential clinical applications in cardiology. Biochim Biophys Acta 2005; 1740:240–248.
Puceat M . Protocols for cardiac differentiation of embryonic stem cells. Methods 2008; 45:168–171.
Fu JD, Yu HM, Wang R, Liang J, Yang HT . Developmental regulation of intracellular calcium transients during cardiomyocyte differentiation of mouse embryonic stem cells. Acta Pharmacol Sin 2006; 27:901–910.
Watanabe K, Ueno M, Kamiya D, et al. A ROCK inhibitor permits survival of dissociated human embryonic stem cells. Nat Biotechnol 2007; 25:681–686.
Wobus AM . Potential of embryonic stem cells. Mol Aspects Med 2001; 22:149–164.
Kattman SJ, Adler ED, Keller GM . Specification of multipotential cardiovascular progenitor cells during embryonic stem cell differentiation and embryonic development. Trends Cardiovasc Med 2007; 17:240–246.
Wu SM, Chien KR, Mummery C . Origins and fates of cardiovascular progenitor cells. Cell 2008; 132:537–543.
Martin-Puig S, Wang Z, Chien KR . Lives of a heart cell: tracing the origins of cardiac progenitors. Cell Stem Cell 2008; 2:320–231.
Christoforou N, Miller RA, Hill CM, et al. Mouse ES cell-derived cardiac precursor cells are multipotent and facilitate identification of novel cardiac genes. J Clin Invest 2008; 118:894–903.
He JQ, Ma Y, Lee Y, Thomson JA, Kamp TJ . Human embryonic stem cells develop into multiple types of cardiac myocytes: action potential characterization. Circ Res 2003; 93:32–39.
Bers DM . Sarcoplasmic reticulum Ca release in intact ventricular myocytes. Front Biosci 2002; 7:d1697–d1711.
Li C, Yang Y, Gu J, Ma Y, Jin Y . Derivation and transcriptional profiling analysis of pluripotent stem cell lines from rat blastocysts. Cell Res 2009; 19:173–186.
Murry CE, Keller G . Differentiation of embryonic stem cells to clinically relevant populations: lessons from embryonic development. Cell 2008; 132:661–680.
Rossant J . Stem cells and early lineage development. Cell 2008; 132:527–531.
Wartenberg M, Gunther J, Hescheler J, Sauer H . The embryoid body as a novel in vitro assay system for antiangiogenic agents. Lab Invest 1998; 78:1301–1314.
Harding SE, Ali NN, Brito-Martins M, Gorelik J . The human embryonic stem cell-derived cardiomyocyte as a pharmacological model. Pharmacol Ther 2007; 113:341–353.
Winkler J, Sotiriadou I, Chen S, Hescheler J, Sachinidis A . The potential of embryonic stem cells combined with -omics technologies as model systems for toxicology. Curr Med Chem 2009; 16:4814–4827.
Desbaillets I, Ziegler U, Groscurth P, Gassmann M . Embryoid bodies: an in vitro model of mouse embryogenesis. Exp Physiol 2000; 85:645–651.
Itskovitz-Eldor J, Schuldiner M, Karsenti D, et al. Differentiation of human embryonic stem cells into embryoid bodies compromising the three embryonic germ layers. Mol Med 2000; 6:88–95.
Kurosawa H . Methods for inducing embryoid body formation: in vitro differentiation system of embryonic stem cells. J Biosci Bioeng 2007; 103:389–398.
Ng ES, Davis RP, Azzola L, Stanley EG, Elefanty AG . Forced aggregation of defined numbers of human embryonic stem cells into embryoid bodies fosters robust, reproducible hematopoietic differentiation. Blood 2005; 106:1601–1603.
Wang X, Wei G, Yu W, et al. Scalable producing embryoid bodies by rotary cell culture system and constructing engineered cardiac tissue with ES-derived cardiomyocytes in vitro. Biotechnol Prog 2006; 22:811–818.
Burridge PW, Anderson D, Priddle H, et al. Improved human embryonic stem cell embryoid body homogeneity and cardiomyocyte differentiation from a novel V-96 plate aggregation system highlights interline variability. Stem Cells 2007; 25:929–938.
Parmacek MS, Epstein JA . Pursuing cardiac progenitors: regeneration redux. Cell 2005; 120:295–298.
Yang L, Soonpaa MH, Adler ED, et al. Human cardiovascular progenitor cells develop from a KDR+ embryonic-stem-cell-derived population. Nature 2008; 453:524–528.
Bu L, Jiang X, Martin-Puig S, et al. Human ISL1 heart progenitors generate diverse multipotent cardiovascular cell lineages. Nature 2009; 460:113–117.
Hescheler J, Fleischmann BK, Lentini S, et al. Embryonic stem cells: a model to study structural and functional properties in cardiomyogenesis. Cardiovasc Res 1997; 36:149–162.
Fu JD, Li J, Tweedie D, et al. Crucial role of the sarcoplasmic reticulum in the developmental regulation of Ca2+ transients and contraction in cardiomyocytes derived from embryonic stem cells. FASEB J 2006; 20:181–183.
Yang HT, Tweedie D, Wang S, et al. The ryanodine receptor modulates the spontaneous beating rate of cardiomyocytes during development. Proc Natl Acad Sci USA 2002; 99:9225–9230.
Nichols J, Ying QL . Derivation and propagation of embryonic stem cells in serum- and feeder-free culture. Methods Mol Biol 2006; 329:91–98.
Grynkiewicz G, Poenie M, Tsien RY . A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem 1985; 260:3440–3450.
Acknowledgements
The study was supported by grants from the State Major Research Program of China (2007CB947100, 2010CB945603, and 2011CB965301), National Science and Technology Major Project (2009ZX09503-024), Knowledge Innovation Program of CAS (KSCX2-YW-R-233, KSCX1-YW-R-46), Strategic Priority Research Program of CAS (XDA01030000), and the Ministry of Agriculture (2009ZX08010-016B). We thank Zhongyan Chen for performing fetal rat heart isolation, Lan Wu for technique assistance in the measurement of Ca2+ transients, Lei Qian and Jihui Zhang for performing rESCs culture, and Dr Ying Jin for kindly providing the Oct4 antibody.
Author information
Authors and Affiliations
Corresponding authors
Additional information
( Supplementary information is linked to the online version of the paper on the Cell Research website.)
Supplementary information
Supplementary information, Figure S1
Identification of optimal conditions for in vitro differentiation protocol of rESCs. (PDF 215 kb)
Supplementary information, Figure S2
Analysis of the significance of rEFM for cardiac differentiation of rESCs. (PDF 72 kb)
Supplementary information, Figure S3
Comparisons of EB generation methods. (PDF 250 kb)
Supplementary information, Table S1
Primer Sequences for RT- PCR (PDF 6 kb)
Supplementary information, Table S2
Primer Sequences for Q-PCR (PDF 6 kb)
Rights and permissions
About this article
Cite this article
Cao, N., Liao, J., Liu, Z. et al. In vitro differentiation of rat embryonic stem cells into functional cardiomyocytes. Cell Res 21, 1316–1331 (2011). https://doi.org/10.1038/cr.2011.48
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/cr.2011.48
Keywords
This article is cited by
-
MLL3/MLL4 methyltransferase activities control early embryonic development and embryonic stem cell differentiation in a lineage-selective manner
Nature Genetics (2023)
-
Rho Kinases in Embryonic Development and Stem Cell Research
Archivum Immunologiae et Therapiae Experimentalis (2022)
-
Myosin light chain 2 marks differentiating ventricular cardiomyocytes derived from human embryonic stem cells
Pflügers Archiv - European Journal of Physiology (2021)
-
Disruption of histamine/H1R signaling pathway represses cardiac differentiation and maturation of human induced pluripotent stem cells
Stem Cell Research & Therapy (2020)
-
A novel approach to differentiate rat embryonic stem cells in vitro reveals a role for RNF12 in activation of X chromosome inactivation
Scientific Reports (2019)