Abstract
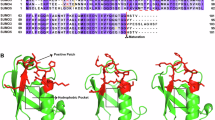
Discovery of emerging REGγ-regulated proteins has accentuated the REGγ-proteasome as an important pathway in multiple biological processes, including cell growth, cell cycle regulation, and apoptosis. However, little is known about the regulation of the REGγ-proteasome pathway. Here we demonstrate that REGγ can be SUMOylated in vitro and in vivo by SUMO-1, SUMO-2, and SUMO-3. The SUMO-E3 protein inhibitor of activated STAT (PIAS)1 physically associates with REGγ and promotes SUMOylation of REGγ. SUMOylation of REGγ was found to occur at multiple sites, including K6, K14, and K12. Mutation analysis indicated that these SUMO sites simultaneously contributed to the SUMOylation status of REGγ in cells. Posttranslational modification of REGγ by SUMO conjugation was revealed to mediate cytosolic translocation of REGγ and to cause increased stability of this proteasome activator. SUMOylation-deficient REGγ displayed attenuated ability to degrade p21Waf//Cip1 due to reduced affinity of the REGγ SUMOylation-defective mutant for p21. Taken together, we report a previously unrecognized mechanism regulating the activity of the proteasome activator REGγ. This regulatory mechanism may enable REGγ to function as a more potent factor in protein degradation with a broader substrate spectrum.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Chen X, Barton LF, Chi Y, Clurman BE, Roberts JM . Ubiquitin-independent degradation of cell-cycle inhibitors by the REGgamma proteasome. Mol Cell 2007; 26:843–852.
Li X, Amazit L, Long W, Lonard DM, Monaco JJ, O'Malley BW . Ubiquitin- and ATP-independent proteolytic turnover of p21 by the REGgamma-proteasome pathway. Mol Cell 2007; 26:831–842.
Li X, Lonard D, Jung S, et al. The SRC-3/AIB1 coactivator is degraded in a ubiquitin-and ATP-independent manner by the REG [gamma] proteasome. Cell 2006; 124:381–392.
Mao I, Liu J, Li X, Luo H . REGgamma, a proteasome activator and beyond? Cell Mol Life Sci 2008; 65:3971–3980.
Roessler M, Rollinger W, Mantovani-Endl L, et al. Identification of PSME3 as a novel serum tumor marker for colorectal cancer by combining two-dimensional polyacrylamide gel electrophoresis with a strictly mass spectrometry-based approach for data analysis. Mol Cell Proteomics 2006; 5:2092.
Yu G, Zhao Y, He J et al. Comparative analysis of REG{gamma} expression in mouse and human tissues. J Mol Cell Biol 2010; 2:192–198.
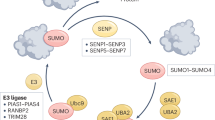
Yeh ET . SUMOylation and De-SUMOylation: wrestling with life's processes. J Biol Chem 2009; 284:8223–8227.
Wang Y, Dasso M . SUMOylation and deSUMOylation at a glance. J Cell Sci 2009; 122:4249.
Geiss-Friedlander R, Melchior F . Concepts in sumoylation: a decade on. Nat Rev Mol Cell Biol 2007; 8:947–956.
Yurchenko V, Xue Z, Sadofsky M . SUMO modification of human XRCC4 regulates its localization and function in DNA double-strand break repair. Mol Cell Biol 2006; 26:1786.
Matunis MJ, Coutavas E, Blobel G . A novel ubiquitin-like modification modulates the partitioning of the Ran-GTPase-activating protein RanGAP1 between the cytosol and the nuclear pore complex. J Cell Biol 1996; 135:1457–1470.
Desterro JM, Rodriguez MS, Hay RT . SUMO-1 modification of IkappaBalpha inhibits NF-kappaB activation. Mol Cell 1998; 2:233–239.
Zhang Z, Zhang R . Proteasome activator PA28 gamma regulates p53 by enhancing its MDM2-mediated degradation. EMBO J 2008; 27:852–864.
Rechsteiner M, Hill CP . Mobilizing the proteolytic machine: cell biological roles of proteasome activators and inhibitors. Trends Cell Biol 2005; 15:27–33.
Miyauchi Y, Yogosawa S, Honda R, Nishida T, Yasuda H . Sumoylation of Mdm2 by protein inhibitor of activated STAT (PIAS) and RanBP2 enzymes. J Biol Chem 2002; 277:50131–50136.
Tan JA, Song J, Chen Y, Durrin LK . Phosphorylation-dependent interaction of SATB1 and PIAS1 directs SUMO-regulated caspase cleavage of SATB1. Mol Cell Biol 2010; 30:2823–2836.
Galanty Y, Belotserkovskaya R, Coates J, Polo S, Miller KM, Jackson SP . Mammalian SUMO E3-ligases PIAS1 and PIAS4 promote responses to DNA double-strand breaks. Nature 2009; 462:935–939.
Kahyo T, Nishida T, Yasuda H . Involvement of PIAS1 in the sumoylation of tumor suppressor p53. Mol Cell 2001; 8:713–718.
Lin D, Huang Y, Jeng J, et al. Role of SUMO-interacting motif in Daxx SUMO modification, subnuclear localization, and repression of SUMOylated transcription factors. Mol Cell 2006; 24:341–354.
Figueroa-Romero C, Iniguez-Lluhi JA, Stadler J, et al. SUMOylation of the mitochondrial fission protein Drp1 occurs at multiple nonconsensus sites within the B domain and is linked to its activity cycle. FASEB J 2009; 23:3917–3927.
Wood LD, Irvin BJ, Nucifora G, Luce KS, Hiebert SW . Small ubiquitin-like modifier conjugation regulates nuclear export of TEL, a putative tumor suppressor. Proc Natl Acad Sci USA 2003; 100:3257–3262.
Cheng J, Kang X, Zhang S, Yeh ET . SUMO-specific protease 1 is essential for stabilization of HIF1alpha during hypoxia. Cell 2007; 131:584–595.
Liang M, Melchior F, Feng X, Lin X . Regulation of Smad4 sumoylation and transforming growth factor-beta signaling by protein inhibitor of activated STAT1. J Biol Chem 2004; 279:22857–22865.
Verger A, Perdomo J, Crossley M . Modification with SUMO. A role in transcriptional regulation. EMBO Rep 2003; 4:137–142.
Acknowledgements
This work was supported by the National Institutes of Health (1R01CA131914). This manuscript was also funded in part by the National Natural Science Foundation of China (30811120435, 30870503, 81071657), the Science and Technology Commission of Shanghai Municipality (06DZ22923, 08PJ14047, 10JC1404200, 09ZZ41), and the National Basic Research Program (2009CB918402, 2011CB504200).
Author information
Authors and Affiliations
Corresponding author
Additional information
( Supplementary information is linked to the online version of the paper on the Cell Research website.)
Supplementary information
Supplementary information, Figure S1
GFP can not be SUMOylated. (PDF 89 kb)
Supplementary information, Figure S2
REGγ can be SUMOylated by SUMO-1, -2, and -3. (PDF 77 kb)
Supplementary information, Figure S3
PIAS1 is the major SUMO-E3 ligase for REGγ. (PDF 147 kb)
Supplementary information, Figure S4
REGγ-SUMO fusion proteins are capable of cytoplasmic translocation. (PDF 91 kb)
Supplementary information, Figure S5
Evolutionary view of the putative SUMOylation sites in REGγ. (PDF 175 kb)
Supplementary information, Figure S6
REGγ SUMOylation occurs on multiple sites. (PDF 146 kb)
Supplementary information, Figure S7
SUMO modification enhances REGγ stability. (PDF 83 kb)
Supplementary information, Figure S8
Endogenous p21 turnover is increased in hyper-SUMOylated cell. (PDF 166 kb)
Supplementary information, Table S1
Predicted REGγ SUMOylation sites with SUMOplot (PDF 1027 kb)
Supplementary information, Table S2
Predicted REGγ SUMOylation sites with SUMOsp2.0 (PDF 1108 kb)
Rights and permissions
About this article
Cite this article
Wu, Y., Wang, L., Zhou, P. et al. Regulation of REGγ cellular distribution and function by SUMO modification. Cell Res 21, 807–816 (2011). https://doi.org/10.1038/cr.2011.57
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/cr.2011.57
Keywords
This article is cited by
-
Cold temperature extends longevity and prevents disease-related protein aggregation through PA28γ-induced proteasomes
Nature Aging (2023)
-
Identification of a BRAF/PA28γ/MEK1 signaling axis and its role in epithelial-mesenchymal transition in oral submucous fibrosis
Cell Death & Disease (2022)
-
SUMO3 modification by PIAS1 modulates androgen receptor cellular distribution and stability
Cell Communication and Signaling (2019)
-
PA28gamma emerges as a novel functional target of tumour suppressor microRNA-7 in non-small-cell lung cancer
British Journal of Cancer (2014)