Abstract
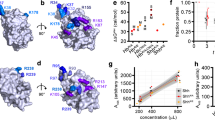
Brachydactyly type A1 (BDA1), the first recorded Mendelian autosomal dominant disorder in humans, is characterized by a shortening or absence of the middle phalanges. Heterozygous missense mutations in the Indian Hedgehog (IHH) gene have been identified as a cause of BDA1; however, the biochemical consequences of these mutations are unclear. In this paper, we analyzed three BDA1 mutations (E95K, D100E, and E131K) in the N-terminal fragment of Indian Hedgehog (IhhN). Structural analysis showed that the E95K mutation changes a negatively charged area to a positively charged area in a calcium-binding groove, and that the D100E mutation changes the local tertiary structure. Furthermore, we showed that the E95K and D100E mutations led to a temperature-sensitive and calcium-dependent instability of IhhN, which might contribute to an enhanced intracellular degradation of the mutant proteins via the lysosome. Notably, all three mutations affected Hh binding to the receptor Patched1 (PTC1), reducing its capacity to induce cellular differentiation. We propose that these are common features of the mutations that cause BDA1, affecting the Hh tertiary structure, intracellular fate, binding to the receptor/partners, and binding to extracellular components. The combination of these features alters signaling capacity and range, but the impact is likely to be variable and mutation-dependent. The potential variation in the signaling range is characterized by an enhanced interaction with heparan sulfate for IHH with the E95K mutation, but not the E131K mutation. Taken together, our results suggest that these IHH mutations affect Hh signaling at multiple levels, causing abnormal bone development and abnormal digit formation.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Ingham PW, McMahon AP . Hedgehog signaling in animal development: paradigms and principles. Genes Dev 2001; 15:3059–3087.
McMahon AP, Ingham PW, Tabin CJ . Developmental roles and clinical significance of hedgehog signaling. Curr Top Dev Biol 2003; 53:1–114.
Lee JJ, Ekker SC, von Kessler DP, Porter JA, Sun BI, Beachy PA . Autoproteolysis in hedgehog protein biogenesis. Science 1994; 266:1528–1537.
Porter JA, von Kessler DP, Ekker SC, et al. The product of hedgehog autoproteolytic cleavage active in local and long-range signalling. Nature 1995; 374:363–366.
Porter JA, Ekker SC, Park WJ, et al. Hedgehog patterning activity: role of a lipophilic modification mediated by the carboxy-terminal autoprocessing domain. Cell 1996; 86:21–34.
Burke R, Nellen D, Bellotto M, et al. Dispatched, a novel sterol-sensing domain protein dedicated to the release of cholesterol-modified hedgehog from signaling cells. Cell 1999; 99:803–815.
Fuse N, Maiti T, Wang B, et al. Sonic hedgehog protein signals not as a hydrolytic enzyme but as an apparent ligand for patched. Proc Natl Acad Sci USA 1999; 96:10992–10999.
Incardona JP, Lee JH, Robertson CP, Enga K, Kapur RP, Roelink H . Receptor-mediated endocytosis of soluble and membrane-tethered Sonic hedgehog by Patched-1. Proc Natl Acad Sci USA 2000; 97:12044–12049.
Bellaiche Y, The I, Perrimon N . Tout-velu is a Drosophila homologue of the putative tumour suppressor EXT-1 and is needed for Hh diffusion. Nature 1998; 394:85–88.
Takei Y, Ozawa Y, Sato M, Watanabe A, Tabata T . Three Drosophila EXT genes shape morphogen gradients through synthesis of heparan sulfate proteoglycans. Development 2004; 131:73–82.
Han C, Belenkaya TY, Khodoun M, Tauchi M, Lin X, Lin X . Distinct and collaborative roles of Drosophila EXT family proteins in morphogen signalling and gradient formation. Development 2004; 131:1563–1575.
Zeng X, Goetz JA, Suber LM, Scott WJ Jr, Schreiner CM Robbins DJ . A freely diffusible form of Sonic hedgehog mediates long-range signalling. Nature 2001; 411:716–720.
Chen MH, Li YJ, Kawakami T, Xu SM, Chuang PT . Palmitoylation is required for the production of a soluble multimeric Hedgehog protein complex and long-range signaling in vertebrates. Genes Dev 2004; 18:641–659.
Feng J, White B, Tyurina OV, et al. Synergistic and antagonistic roles of the Sonic hedgehog N- and C-terminal lipids. Development 2004; 131:4357–4370.
Roessler E, Belloni E, Gaudenz K, et al. Mutations in the human Sonic Hedgehog gene cause holoprosencephaly. Nature Genet 1996; 14:357–360.
Roessler E, Belloni E, Gaudenz K, et al. Mutations in the C-terminal domain of Sonic Hedgehog cause holoprosencephaly. Hum Mol Genet 1997; 6:1847–1853.
Gao B, Guo J, She C, et al. Mutations in IHH, encoding Indian hedgehog, cause brachydactyly type A-1. Nat Genet 2001; 28:386–388.
Gao B, He L . Answering a century old riddle: brachydactyly type A1. Cell Res 2004; 14:179–187.
Farabee WC . Hereditary and sexual influence in meristic variation: a study of digital malformations in man. Thesis, Harvard University, 1903.
Lanske B, Karaplis AC, Lee K, et al. PTH/PTHrP receptor in early development and Indian hedgehog-regulated bone growth. Science 1996; 273:663–666.
Kirkpatrick TJ, Au KS, Mastrobattista JM, McCready ME, Bulman DE, Northrup H . Identification of a mutation in the Indian hedgehog (IHH) gene causing brachydactyly type A1 and evidence for a third locus. J Med Genet 2003; 40:42–44.
McCready ME, Sweeney E, Fryer AE, et al. A novel mutation in the IHH gene causes brachydactyly type A1: a 95-year-old mystery resolved. Hum Genet 2002; 111:368–375.
Giordano N, Gennari L, Bruttini M, et al. Mild brachydactyly type A1 maps to chromosome 2q35-q36 and is caused by a novel IHH mutation in a three generation family. J Med Genet 2003; 40:132–135.
Lodder EM, Hoogeboom AJM, Coert JH, de Graaff E . Deletion of 1 amino acid in Indian hedgehog leads to brachydactylyA1. Am J Med Genet Part A 2008; 146:2152–2154.
Hellemans J, Coucke PJ, Giedion A, et al. Homozygous mutations in IHH cause acrocapitofemoral dysplasia, an autosomal recessive disorder with cone-shaped epiphyses in hands and hips. Am J Hum Genet 2003; 72:1040–1046.
McLellan JS, Zheng X, Hauk G, Ghirlando R, Beachy PA, Leahy DJ . The mode of Hedgehog binding to Ihog homologues is not conserved across different phyla. Nature 2008; 455:979–983.
Gao B, Hu JX, Stricker S, et al. A mutation in Ihh that causes digit abnormalities alters its signalling capacity and range. Nature 2009; 458:1196–1200.
Hall TM, Porter JA, Beachy PA, Leahy DJ . A potential catalytic site revealed by the 1.7-A crystal structure of the amino-terminal signaling domain of sonic hedgehog. Nature 1995; 378:212–216.
Bishop B, Aricescu AR, Harlos K, O'Callaghan CA, Jones EY, Siebold C . Structural insights into hedgehog ligand sequestration by the human hedgehog-interacting protein HHIP. Nat Struct Mol Biol 2009; 16:698–703.
Kavran JM, Ward MD, Oladosu OO, Mulepati S, Leahy DJ . All mammalian Hedgehog proteins interact with cell adhesion molecule, down-regulated by oncogenes (CDO) and brother of CDO (BOC) in a conserved manner. J Biol Chem 2010; 285:24584–24590.
DeLano WL . The PyMOL Molecular Graphics System. DeLano Scientific, San Carlos, CA, USA. 2002.
Baker NA, Sept D, Joseph S, Holst MJ, McCammon JA . Electrostatics of nanosystems: application to microtubules and the ribosome. Proc Natl Acad Sci USA 2001; 98:10037–10041.
Peters C, Wolf A, Wagner M, Kuhlmann J, Waldmann H . The cholesterol membrane anchor of the Hedgehog protein confers stable membrane association to lipid-modified proteins. Proc Natl Acad Sci USA 2004; 101:8531–8536.
Maity T, Fuse N, Beachy PA . Molecular mechanisms of Sonic hedgehog mutant effects in holoprosencephaly. Proc Natl Acad Sci USA 2005; 102:17026–17031.
Chen Y, Struhl G . Dual roles for patched in sequestering and transducing Hedgehog. Cell 1996; 87:553–563.
Goetz JA, Singh S, Suber LM, Kull FJ, Robbins DJ . A highly conserved amino-terminal region of sonic hedgehog is required for the formation of its freely diffusible multimeric form. J Biol Chem 2006; 281:4087–4093.
Guo S, Zhou J, Gao B, et al. Missense mutations in IHH impair Indian Hedgehog signaling in C3H10T1/2 cells: implications for brachydactyly type A1, and new targets for Hedgehog signaling. Cell Mol Biol Lett 2010; 15:153–176.
Koziel L, Kunath M, Kelly OG, Vortkamp A . Ext1-dependent heparan sulfate regulates the range of Ihh signaling during endochondral ossification. Dev Cell 2004; 6:801–813.
Rubin JB, Choi Y, Segal RA . Cerebellar proteoglycans regulate sonic hedgehog responses during development. Development 2002; 129:2223–2232.
Capurro MI, Xu P, Shi W, Li F, Jia A, Filmus J . Glypican-3 inhibits Hedgehog signaling during development by competing with patched for Hedgehog binding. Dev Cell 2008; 14:700–711.
Panáková D, Sprong H, Marois E, Thiele C, Eaton S . Lipoprotein particles are required for Hedgehog and Wingless signaling. Nature 2005; 435:58–65.
Eugster C, Panáková D, Mahmoud A, Eaton S . Lipoprotein-heparan sulfate interactions in the Hh pathway. Dev Cell 2007; 13:57–71.
Vyas N, Goswami D, Manonmani A, et al. Nanoscale organization of Hedgehog is essential for long-range signaling. Cell 2008; 133:1214–1227.
Knecht E, Aguado C, Cárcel J, et al. Intracellular protein degradation in mammalian cells: recent developments. Cell Mol Life Sci 2009; 66:2427–2443.
Rubinsztein DC . The roles of intracellular protein-degradation pathways in neurodegeneration. Nature 2006; 443:780–786.
Costelli P, Reffo P, Penna F, Autelli R, Bonelli G, Baccino FM . Ca(2+)-dependent proteolysis in muscle wasting. Int J Biochem Cell Biol 2005; 37:2134–2146.
Ma Y, Erkner A, Gong R, et al. Hedgehog-mediated patterning of the mammalian embryo requires transporter-like function of dispatched. Cell 2002; 111:63–75.
Guy RK . Inhibition of sonic hedgehog autoprocessing in cultured mammalian cells by sterol deprivation. Proc Natl Acad Sci USA 2000; 97:7307–7312.
Cheah KS, Levy A, Trainor PA . et al. Human COL2A1-directed SV40 T antigen expression in transgenic and chimeric mice results in abnormal skeletal development. J Cell Biol 1995; 128:223–237.
Bitgood MJ, McMahon AP . Hedgehog and Bmp genes are coexpressed at many diverse sites of cell–cell interaction in the mouse embryo. Dev Biol 1995; 172:126–138.
Leslie AG . The integration of macromolecular diffraction data. Acta Crystallogr D Biol Crystallogr 2006; 62:48–57.
McCoy AJ . Solving structures of protein complexes by molecular replacement with Phaser. Acta Crystallogr D Biol Crystallogr 2007; 63:32–41.
Emsley P, Cowtan K . Coot: model-building tools for molecular graphics. Acta Crystallogr D Biol Crystallogr 2004; 60:2126–2132.
Murshudov GN, Vagin AA, Dodson EJ . Refinement of macromolecular structures by the maximum-likelihood method. Acta Crystallogr D 1997; 53:240–255.
Brunger AT, Adams PD, Clore GM . et al. Crystallography & NMR system: A new software suite for macromolecular structure determination. Acta Crystallogr D Biol Crystallogr 1998; 54:905–921.
Laskowski RA, MacArthur MW, Moss DS, Thornton JM . PROCHECK: a program to check the stereochemical quality of protein structures. J Appl Cryst 1993; 26:283–291.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (30800613), the 973 Program (2010CB529600, 2007CB947300), the 863 Program (2009AA022701), the Shanghai Municipal Commission of Science and Technology Program (09DJ1400601), the Natural Science Foundation of Shanghai, China (Grant No. 08ZR1411000), the National Key Project for the Investigation of New Drugs (2008ZX09312-003), the Shanghai Leading Academic Discipline Project (B205), and the General Research Fund of Hong Kong (HKU760608M). The co-author, Xizhi Guo, was supported by the “Pujiang Talent” Project (08PJ1407200).
Author information
Authors and Affiliations
Corresponding authors
Additional information
(Supplementary information is linked to the online version of the paper on the Cell Research website.)
Supplementary information
Supplementary information, Table S1
Data collection and refinement statistics of IHH structure (PDF 96 kb)
Supplementary information, Figure S1
The subcellular localization of WT and mutant IhhN in the EcR-CHO cells. (PDF 207 kb)
Supplementary information, Figure S2
The stability of WT and mutant IhhN proteins. (PDF 199 kb)
Rights and permissions
About this article
Cite this article
Ma, G., Yu, J., Xiao, Y. et al. Indian hedgehog mutations causing brachydactyly type A1 impair Hedgehog signal transduction at multiple levels. Cell Res 21, 1343–1357 (2011). https://doi.org/10.1038/cr.2011.76
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/cr.2011.76
Keywords
This article is cited by
-
Suppression of apoptosis impairs phalangeal joint formation in the pathogenesis of brachydactyly type A1
Nature Communications (2024)
-
Altered microRNAs in C3H10T1/2 cells induced by p.E95K mutant IHH signaling
Hereditas (2021)
-
A novel variant of IHH in a Chinese family with brachydactyly type 1
BMC Medical Genetics (2020)
-
RNA-seq reveals downregulated osteochondral genes potentially related to tibia bacterial chondronecrosis with osteomyelitis in broilers
BMC Genetics (2020)
-
p.E95K mutation in Indian hedgehog causing brachydactyly type A1 impairs IHH/Gli1 downstream transcriptional regulation
BMC Genetics (2019)