Abstract
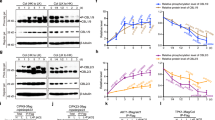
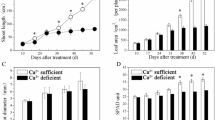
Plant responses to developmental and environmental cues are often mediated by calcium (Ca2+) signals that are transmitted by diverse calcium sensors. The calcineurin B-like (CBL) protein family represents calcium sensors that decode calcium signals through specific interactions with a group of CBL-interacting protein kinases. We report functional analysis of Arabidopsis CBL2 and CBL3, two closely related CBL members that are localized to the vacuolar membrane through the N-terminal tonoplast-targeting sequence. While cbl2 or cbl3 single mutant did not show any phenotypic difference from the wild type, the cbl2 cbl3 double mutant was stunted with leaf tip necrosis, underdeveloped roots, shorter siliques and fewer seeds. These defects were reminiscent of those in the vha-a2 vha-a3 double mutant deficient in vacuolar H+-ATPase (V-ATPase). Indeed, the V-ATPase activity was reduced in the cbl2 cbl3 double mutant, connecting tonoplast CBL-type calcium sensors to the regulation of V-ATPase. Furthermore, cbl2 cbl3 double mutant was compromised in ionic tolerance and micronutrient accumulation, consistent with the defect in V-ATPase activity that has been shown to function in ion compartmentalization. Our results suggest that calcium sensors CBL2 and CBL3 serve as molecular links between calcium signaling and V-ATPase, a central regulator of intracellular ion homeostasis.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Sanders D, Pelloux J, Brownlee C, Harper JF . Calcium at the crossroads of signaling. Plant Cell 2002; 14:S401–S417.
Kudla J, Batistič O, Hashimoto K . Calcium signals: the lead currency of plant information processing. Plant Cell 2010; 22:541–563.
Dodd AN, Kudla J, Sanders D . The language of calcium signaling. Annu Rev Plant Biol 2010; 61:593–620.
Hetherington AM, Brownlee C . The generation of Ca2+ signals in plants. Annu Rev Plant Biol 2004; 55:401–427.
McAinsh MR, Pittman JK . Shaping the calcium signature. New Phytol 2009; 181:275–294.
Luan S, Kudla J, Rodriguez-Concepcion M, Yalovsky S, Gruissem W . Calmodulins and calcineurin B-like proteins: calcium sensors for specific signal response coupling in plants. Plant Cell 2002; 14:S389–S400.
Weinl S, Kudla J . The CBL-CIPK Ca2+-decoding network: function and perspectives. New Phytol 2009; 184:517–528.
Luan S . The CBL-CIPK network in plant calcium signaling. Trends Plant Sci 2009; 14:37–42.
DeFalco TA, Bender KW, Snedden WA . Breaking the code: Ca2+ sensors in plant signalling. Biochem J 2010; 425:27–40.
Kudla J, Xu Q, Harter K, Gruissem W, Luan S . Genes for calcineurin B-like proteins in Arabidopsis are differentially regulated by stress signals. Proc Natl Acad Sci USA 1999; 96:4718–4723.
Liu J, Zhu JK . A calcium sensor homolog required for plant salt tolerance. Science 1998; 280:1943–1945.
Shi J, Kim KN, Ritz O, et al. Novel protein kinases associated with calcineurin B-like calcium sensors in Arabidopsis. Plant Cell 1999; 11:2393–2405.
Guo Y, Halfter U, Ishitani M, Zhu JK . Molecular characterization of functional domains in the protein kinase SOS2 that is required for plant salt tolerance. Plant Cell 2001; 13:1383–1400.
Albrecht V, Ritz O, Linder S, Harter K, Kudla J . The NAF domain defines a novel protein-protein interaction module conserved in Ca2+-regulated kinases. EMBO J 2001; 20:1051–1063.
Luan S, Lan W, Lee SC . Potassium nutrition, sodium toxicity and calcium signaling: connections through the CBL-CIPK network. Curr Opin Plant Biol 2009; 12:339–346.
Liu J, Ishitani M, Halfter U, Kim CS, Zhu JK . The Arabidopsis thaliana SOS2 gene encodes a protein kinase that is required for salt tolerance. Proc Natl Acad Sci USA 2000; 97:3730–3734.
Qiu QS, Guo Y, Dietrich MA, Schumaker KS, Zhu JK . Regulation of SOS1, a plasma membrane Na+/H+ exchanger in Arabidopsis thaliana, by SOS2 and SOS3. Proc Natl Acad Sci USA 2002; 99:8436–8441.
Quintero FJ, Ohta M, Shi H, Zhu JK, Pardo JM . Reconstitution in yeast of the Arabidopsis SOS signaling pathway for Na+ homeostasis. Proc Natl Acad Sci USA 2002; 99:9061–9066.
Quan R, Lin H, Mendoza I, et al. SCABP8/CBL10, a putative calcium sensor, interacts with the protein kinase SOS2 to protect Arabidopsis shoots from salt stress. Plant Cell 2007; 19:1415–1431.
Kim BG, Waadt R, Cheong YH, et al. The calcium sensor CBL10 mediates salt tolerance by regulating ion homeostasis in Arabidopsis. Plant J 2007; 52:473–484.
Xu J, Li HD, Chen LQ, et al. A protein kinase, interacting with two calcineurin B-like proteins, regulates K+ transporter AKT1 in Arabidopsis. Cell 2006; 125:1347–1360.
Li L, Kim BG, Cheong YH, Pandey GK, Luan S . A Ca2+ signaling pathway regulates a K+ channel for low-K response in Arabidopsis. Proc Natl Acad Sci USA 2006; 103:12625–12630.
Cheong YH, Pandey GK, Grant JJ, et al. Two calcineurin B-like calcium sensors, interacting with protein kinase CIPK23, regulate leaf transpiration and root potassium uptake in Arabidopsis. Plant J 2007; 52:223–239.
Ho CH, Lin S, Hu HC, Tsay YF . CHL1 functions as a nitrate sensor in plants. Cell 2009; 138:1184–1194.
Albrecht V, Weinl S, Blazevic D, et al. The calcium sensor CBL1 integrates plant responses to abiotic stresses. Plant J 2003; 36:457–470.
Cheong YH, Kim KN, Pandey GK, Gupta R, Grant JJ, Luan S . CBL1, a calcium sensor that differentially regulates salt, drought, and cold responses in Arabidopsis. Plant Cell 2003; 15:1833–1845.
Pandey GK, Cheong YH, Kim KN, et al. The calcium sensor calcineurin B-like 9 modulates abscisic acid sensitivity and biosynthesis in Arabidopsis. Plant Cell 2004; 16:1912–1924.
Batistič O, Waadt R, Steinhorst L, Held K, Kudla J . CBL-mediated targeting of CIPKs facilitates the decoding of calcium signals emanating from distinct cellular stores. Plant J 2010; 61:211–222.
Waadt R, Schmidt LK, Lohse M, Hashimoto K, Bock R, Kudla J . Multicolor bimolecular fluorescence complementation reveals simultaneous formation of alternative CBL/CIPK complexes in planta. Plant J 2008; 56:505–516.
Batistič O, Sorek N, Schultke S, Yalovsky S, Kudla J . Dual fatty acyl modification determines the localization and plasma membrane targeting of CBL/CIPK Ca2+ signaling complexes in Arabidopsis. Plant Cell 2008; 20:1346–1362.
Kolukisaoglu Ü, Weinl S, Blazevic D, Batistič O, Kudla J . Calcium sensors and their interacting protein kinases: genomics of the Arabidopsis and rice CBL-CIPK signaling networks. Plant Physiol 2004; 134:43–58.
Krebs M, Held K, Binder A, et al. FRET-based genetically encoded sensors allow high-resolution live cell imaging of Ca2+ dynamics. Plant J 2012; 69:181–192.
Nagae M, Nozawa A, Koizumi N, et al. The crystal structure of the novel calcium-binding protein AtCBL2 from Arabidopsis thaliana. J Biol Chem 2003; 278:42240–42246.
Krebs M, Beyhl D, Görlich E, et al. Arabidopsis V-ATPase activity at the tonoplast is required for efficient nutrient storage but not for sodium accumulation. Proc Natl Acad Sci USA 2010; 107:3251–3256.
Schumacher K, Vafeados D, McCarthy M, Sze H, Wilkins T, Chory J . The Arabidopsis det3 mutant reveals a central role for the vacuolar H+-ATPase in plant growth and development. Genes Dev 1999; 13:3259–3270.
Brüx A, Liu TY, Krebs M, et al. Reduced V-ATPase activity in the trans-Golgi network causes oxylipin-dependent hypocotyl growth inhibition in Arabidopsis. Plant Cell 2008; 20:1088–1100.
Sze H, Schumacher K, Müller ML, Padmanaban S, Taiz L . A simple nomenclature for a complex proton pump: VHA genes encode the vacuolar H+-ATPase. Trends Plant Sci 2002; 7:157–161.
Schumacher K, Krebs M . The V-ATPase: small cargo, large effects. Curr Opin Plant Biol 2010; 13:724–730.
Gaxiola RA, Palmgren MG, Schumacher K . Plant proton pumps. FEBS Lett 2007; 581:2204–2214.
Swanson SJ, Jones RL . Gibberellic acid induces vacuolar acidification in barley aleurone. Plant Cell 1996; 8:2211–2221.
Murashige T, Skoog F . A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant 1962; 15:473–495.
Apse MP, Aharon GS, Snedden WA, Blumwald E . Salt tolerance conferred by overexpression of a vacuolar Na+/H+ antiport in Arabidopsis. Science 1999; 285:1256–1258.
Yokoi S, Quintero FJ, Cubero B, et al. Differential expression and function of Arabidopsis thaliana NHX Na+/H+ antiporters in the salt stress response. Plant J 2002; 30:529–539.
Lahner B, Gong J, Mahmoudian M, et al. Genomic scale profiling of nutrient and trace elements in Arabidopsis thaliana. Nat Biotechnol 2003; 21:1215–1221.
Geiger D, Becker D, Vosloh D, et al. Heteromeric AtKC1·AKT1 channels in Arabidopsis roots facilitate growth under K+-limiting conditions. J Biol Chem 2009; 284:21288–21295.
Batistič O, Rehers M, Akerman A, et al. S-acylation-dependent association of the calcium sensor CBL2 with the vacuolar membrane is essential for proper abscisic acid responses. Cell Res 2012; 22:1155–1168.
Bottanelli F, Foresti O, Hanton S, Denecke J . Vacuolar transport in tobacco leaf epidermis cells involves a single route for soluble cargo and multiple routes for membrane cargo. Plant Cell 2011; 23:3007–3025.
Cheng NH, Pittman JK, Shigaki T, et al. Functional association of Arabidopsis CAX1 and CAX3 is required for normal growth and ion homeostasis. Plant Physiol 2005; 138:2048–2060.
Padmanaban S, Lin X, Perera I, Kawamura Y, Sze H . Differential expression of vacuolar H+-ATPase subunit c genes in tissues active in membrane trafficking and their roles in plant growth as revealed by RNAi. Plant Physiol 2004; 134:1514–1526.
Batelli G, Verslues PE, Agius F, et al. SOS2 promotes salt tolerance in part by interacting with the vacuolar H+-ATPase and upregulating its transport activity. Mol Cell Biol 2007; 27:7781–7790.
Leidi EO, Barragán V, Rubio L, et al. The AtNHX1 exchanger mediates potassium compartmentation in vacuoles of transgenic tomato. Plant J 2010; 61:495–506.
Bassil E, Tajima H, Liang YC, et al. The Arabidopsis Na+/H+ antiporters NHX1 and NHX2 control vacuolar pH and K+ homeostasis to regulate growth, flower development, and reproduction. Plant Cell 2011; 23:3482–3497.
Barragán V, Leidi EO, Andrés Z, et al. Ion exchangers NHX1 and NHX2 mediate active potassium uptake into vacuoles to regulate cell turgor and stomatal function in Arabidopsis. Plant Cell 2012; 24:1127–1142.
Yamaguchi T, Aharon GS, Sottosanto JB, Blumwald E . Vacuolar Na+/H+ antiporter cation selectivity is regulated by calmodulin from within the vacuole in a Ca2+- and pH-dependent manner. Proc Natl Acad Sci USA 2005; 102:16107–16112.
Barkla RJ, Vera-Estrella R, Maldonado-Gama M, Pantoja O . Abscisic acid induction of vacuolar H+-ATPase activity in Mesembryanthemum crystallinum is developmentally regulated. Plant Physiol 1999; 120:811–820.
Kluge C, Lahr J, Hanitzsch M, Bolte S, Golldack D, Dietz KJ . New insight into the structure and regulation of the plant vacuolar H+-ATPase. J Bioenerg Biomembr 2003; 35:377–388.
Clough SJ, Bent AF . Floral dip: A simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J 1998; 16:735–743.
Jefferson RA, Kavanagh TA, Bevan MW . GUS fusion: β-Glucurodinase as a sensitive and versatile gene fusion marker in higher plants. EMBO J 1987; 6:3901–3907.
Yoo SD, Cho YH, Sheen J . Arabidopsis mesophyll protoplasts: a versatile cell system for transient gene expression analysis. Nat Protoc 2007; 2:1565–1572.
Yang Q, Chen ZZ, Zhou XF, et al. Overexpression of SOS (Salt Overly Sensitive) genes increases salt tolerance in transgenic Arabidopsis. Mol Plant 2009; 2:22–31.
Cheng NH, Pittman JK, Barkla BJ, Shigaki T, Hirschi KD . The Arabidopsis cax1 mutant exhibits impaired ion homeostasis, development, and hormonal responses and reveals interplay among vacuolar transporters. Plant Cell 2003; 15:347–364.
Acknowledgements
We thank Prof Karin Schumacher and Dr Melanie Krebs (University of Heidelberg, Germany) for providing the vha-a2 vha-a3 mutant seeds. Special thanks would go to Chen Zhong (Shanghai Institutes for Biological Sciences, CAS) for the assistance in ionomic analysis. This work is supported by the grants from the National Natural Science Foundation of China (31171169, 30872044), the National mega project of GMO crops (2011ZX08001-003, 2011ZX08004-002 and 2011ZX08010-004) to HXZ and National Science Foundation to SL.
Author information
Authors and Affiliations
Corresponding authors
Additional information
( Supplementary information is linked to the online version of the paper on the Cell Research website.)
Supplementary information
Supplementary information, Figure S1
Expression of CBL2 and CBL3 in response to various abiotic stresses. (PDF 92 kb)
Supplementary information, Figure S2
The N-terminal 16-aa of CBL2 is required but not sufficient for its tonoplast targeting. (PDF 562 kb)
Supplementary information, Figure S3
The first 19-aa in the N-terminus of CBL2 determines the tonoplast localization. (PDF 719 kb)
Supplementary information, Figure S4
The efficacy of CBL2 Tonoplast Targeting Sequence (TTS) is independent of its N-terminal location. (PDF 353 kb)
Supplementary information, Figure S5
TTS targets several soluble proteins but not transmembrane proteins to the tonoplast. (PDF 459 kb)
Supplementary information, Figure S6
Purification of recombinant CBL2 and CBL3 proteins from E-Coli cells. (PDF 191 kb)
Supplementary information, Figure S7
Complementation of the cbl2 cbl3 double mutant by CBL2 or CBL3. (PDF 60 kb)
Supplementary information, Figure S8
Phenotype of cbl2 cbl3 and vha-a2 vha-a3 plants grown in the soil. (PDF 365 kb)
Supplementary information, Figure S9
In situ calibration of BCECF-AM in protoplast vacuoles. (PDF 228 kb)
Supplementary information, Figure S10
Growth phenotype of cbl2, cbl3 single mutants and cbl2 cbl3 double mutant under various ionic stress conditions. (PDF 580 kb)
Supplementary information, Figure S11
Yeast two-hybrid assays on CBL2/3 in combination with VHA subunits. (PDF 58 kb)
Supplementary information, Figure S12
CBL2 and CBL3 interact with an array of CIPK proteins. (PDF 116 kb)
Supplementary information, Table S1
Primers used in this study. (PDF 9 kb)
Supplementary information, Data S1
The flanking sequence of the T-DNA insertion lines in this study. (PDF 11 kb)
Rights and permissions
About this article
Cite this article
Tang, RJ., Liu, H., Yang, Y. et al. Tonoplast calcium sensors CBL2 and CBL3 control plant growth and ion homeostasis through regulating V-ATPase activity in Arabidopsis. Cell Res 22, 1650–1665 (2012). https://doi.org/10.1038/cr.2012.161
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/cr.2012.161
Keywords
This article is cited by
-
Evolution of the CBL and CIPK gene families in Medicago: genome-wide characterization, pervasive duplication, and expression pattern under salt and drought stress
BMC Plant Biology (2022)
-
Isolation of the 3β-HSD promoter from Digitalis ferruginea subsp. ferruginea and its functional characterization in Arabidopsis thaliana
Molecular Biology Reports (2022)
-
Illuminating the cells: transient transformation of citrus to study gene functions and organelle activities related to fruit quality
Horticulture Research (2021)
-
cDNA-AFLP technique discloses differential gene expression in response to salinity in wheat (Triticum aestivum L.)
Genetic Resources and Crop Evolution (2021)
-
A calcium signalling network activates vacuolar K+ remobilization to enable plant adaptation to low-K environments
Nature Plants (2020)