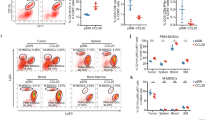
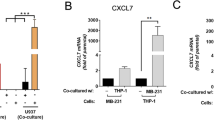
Abstract
CCL5 is a member of the CC chemokine family expressed in a wide array of immune and non-immune cells in response to stress signals. CCL5 expression correlates with advanced human breast cancer. However, its functional significance and mode of action have not been established. Here, we show that CCL5-deficient mice are resistant to highly aggressive, triple-negative mammary tumor growth. Hematopoietic CCL5 is dominant in this phenotype. The absence of hematopoietic CCL5 causes aberrant generation of CD11b+/Gr-1+, myeloid-derived suppressor cells (MDSCs) in the bone marrow in response to tumor growth by accumulating Ly6Chi and Ly6G+ MDSCs with impaired capacity to suppress cytotoxicity of CD8+ T cells. These properties of CCL5 are observed in both orthotopic and spontaneous mammary tumors. Antibody-mediated systemic blockade of CCL5 inhibits tumor progression and enhances the efficacy of therapeutic vaccination against non-immunogenic tumors. CCL5 also helps maintain the immunosuppressive capacity of human MDSCs. Our study uncovers a novel, chemokine-independent activity of the hematopoietically derived CCL5 that promotes mammary tumor progression via generating MDSCs in the bone marrow in cooperation with tumor-derived colony-stimulating factors. The study sheds considerable light on the interplay between the hematopoietic compartment and tumor niche. Because of the apparent dispensable nature of this molecule in normal physiology, CCL5 may represent an excellent therapeutic target in immunotherapy for breast cancer as well as a broad range of solid tumors that have significant amounts of MDSC infiltration.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Appay V, Rowland-Jones SL . RANTES: a versatile and controversial chemokine. Trends Immunol 2001; 22:83–87.
Levy JA . The unexpected pleiotropic activities of RANTES. J Immunol 2009; 182:3945–3946.
Pakianathan DR, Kuta EG, Artis DR, Skelton NJ, Hebert CA . Distinct but overlapping epitopes for the interaction of a CC-chemokine with CCR1, CCR3 and CCR5. Biochemistry 1997; 36:9642–9648.
Movahedi K, Guilliams M, Van den Bossche J, et al. Identification of discrete tumor-induced myeloid-derived suppressor cell subpopulations with distinct T cell-suppressive activity. Blood 2008; 111:4233–4244.
Yaal-Hahoshen N, Shina S, Leider-Trejo L, et al. The chemokine CCL5 as a potential prognostic factor predicting disease progression in stage II breast cancer patients. Clin Cancer Res 2006; 12:4474–4480.
Niwa Y, Akamatsu H, Niwa H, Sumi H, Ozaki Y, Abe A . Correlation of tissue and plasma RANTES levels with disease course in patients with breast or cervical cancer. Clin Cancer Res 2001; 7:285–289.
Nouh MA, Eissa SA, Zaki SA, El-Maghraby SM, Kadry DY . Importance of Serum IL-18 and RANTES as Markers for Breast Carcinoma Progression. J Egypt Natl Canc Inst 2005; 17:51–55.
Bieche I, Lerebours F, Tozlu S, Espie M, Marty M, Lidereau R . Molecular profiling of inflammatory breast cancer: identification of a poor-prognosis gene expression signature. Clin Cancer Res 2004; 10:6789–6795.
Wigler N, Shina S, Kaplan O, et al. Breast carcinoma: a report on the potential usage of the CC chemokine RANTES as a marker for a progressive disease. Isr Med Assoc J 2002; 4:940–943.
Sauer G, Schneiderhan-Marra N, Kazmaier C, et al. Prediction of nodal involvement in breast cancer based on multiparametric protein analyses from preoperative core needle biopsies of the primary lesion. Clin Cancer Res 2008; 14:3345–3353.
Luboshits G, Shina S, Kaplan O, et al. Elevated expression of the CC chemokine regulated on activation, normal T cell expressed and secreted (RANTES) in advanced breast carcinoma. Cancer Res 1999; 59:4681–4687.
Adler EP, Lemken CA, Katchen NS, Kurt RA . A dual role for tumor-derived chemokine RANTES (CCL5). Immunol Lett 2003; 90:187–194.
Robinson SC, Scott KA, Wilson JL, Thompson RG, Proudfoot AE, Balkwill FR . A chemokine receptor antagonist inhibits experimental breast tumor growth. Cancer Res 2003; 63:8360–8365.
Soria G, Ben-Baruch A . The inflammatory chemokines CCL2 and CCL5 in breast cancer. Cancer Lett 2008; 267:271–285.
Jayasinghe MM, Golden JM, Nair P, O'Donnell CM, Werner MT, Kurt RA . Tumor-derived CCL5 does not contribute to breast cancer progression. Breast Cancer Res Treat 2008; 111:511–521.
Ostrand-Rosenberg S, Grusby MJ, Clements VK . Cutting edge: STAT6-deficient mice have enhanced tumor immunity to primary and metastatic mammary carcinoma. J Immunol 2000; 165:6015–6019.
Kelner GS, Kennedy J, Bacon KB, et al. Lymphotactin: a cytokine that represents a new class of chemokine. Science 1994; 266:1395–1399.
Dolcetti L, Peranzoni E, Ugel S, et al. Hierarchy of immunosuppressive strength among myeloid-derived suppressor cell subsets is determined by GM-CSF. Eur J Immunol 2010; 40:22–35.
Sawanobori Y, Ueha S, Kurachi M, et al. Chemokine-mediated rapid turnover of myeloid-derived suppressor cells in tumor-bearing mice. Blood 2008; 111:5457–5466.
Youn JI, Nagaraj S, Collazo M, Gabrilovich DI . Subsets of myeloid-derived suppressor cells in tumor-bearing mice. J Immunol 2008; 181:5791–5802.
Zhu B, Bando Y, Xiao S, et al. CD11b+Ly-6C(hi) suppressive monocytes in experimental autoimmune encephalomyelitis. J Immunol 2007; 179:5228–5237.
Melani C, Chiodoni C, Forni G, Colombo MP . Myeloid cell expansion elicited by the progression of spontaneous mammary carcinomas in c-erbB-2 transgenic BALB/c mice suppresses immune reactivity. Blood 2003; 102:2138–2145.
Ling V, Luxenberg D, Wang J, et al. Structural identification of the hematopoietic progenitor antigen ER-MP12 as the vascular endothelial adhesion molecule PECAM-1 (CD31). Eur J Immunol 1997; 27:509–514.
Gabrilovich DI, Nagaraj S . Myeloid-derived suppressor cells as regulators of the immune system. Nat Rev Immunol 2009; 9:162–174.
Katz BZ, Eshel R, Sagi-Assif O, Witz IP . An association between high Ly-6A/E expression on tumor cells and a highly malignant phenotype. Int J Cancer 1994; 59:684–691.
Trottier MD, Newsted MM, King LE, Fraker PJ . Natural glucocorticoids induce expansion of all developmental stages of murine bone marrow granulocytes without inhibiting function. Proc Natl Acad Sci USA 2008; 105:2028–2033.
de Bruijn MF, Slieker WA, van der Loo JC, Voerman JS, van Ewijk W, Leenen PJ . Distinct mouse bone marrow macrophage precursors identified by differential expression of ER-MP12 and ER-MP20 antigens. Eur J Immunol 1994; 24:2279–2284.
Shojaei F, Wu X, Qu X, et al. G-CSF-initiated myeloid cell mobilization and angiogenesis mediate tumor refractoriness to anti-VEGF therapy in mouse models. Proc Natl Acad Sci USA 2009; 106:6742–6747.
Shojaei F, Wu X, Zhong C, et al. Bv8 regulates myeloid-cell-dependent tumour angiogenesis. Nature 2007; 450:825–831.
Kusmartsev S, Cheng F, Yu B, et al. All-trans-retinoic acid eliminates immature myeloid cells from tumor-bearing mice and improves the effect of vaccination. Cancer Res 2003; 63:4441–4449.
Li Y, Hively WP, Varmus HE . Use of MMTV-Wnt-1 transgenic mice for studying the genetic basis of breast cancer. Oncogene 2000; 19:1002–1009.
Lechner MG, Liebertz DJ, Epstein AL . Characterization of cytokine-induced myeloid-derived suppressor cells from normal human peripheral blood mononuclear cells. J Immunol 2010; 185:2273–2284.
Seung LP, Rowley DA, Dubey P, Schreiber H . Synergy between T-cell immunity and inhibition of paracrine stimulation causes tumor rejection. Proc Natl Acad Sci USA 1995; 92:6254–6258.
Bronte V, Chappell DB, Apolloni E, et al. Unopposed production of granulocyte-macrophage colony-stimulating factor by tumors inhibits CD8+ T cell responses by dysregulating antigen-presenting cell maturation. J Immunol 1999; 162:5728–5737.
Salvadori S, Martinelli G, Zier K . Resection of solid tumors reverses T cell defects and restores protective immunity. J Immunol 2000; 164:2214–2220.
Terabe M, Matsui S, Park JM, et al. Transforming growth factor-beta production and myeloid cells are an effector mechanism through which CD1d-restricted T cells block cytotoxic T lymphocyte-mediated tumor immunosurveillance: abrogation prevents tumor recurrence. J Exp Med 2003; 198:1741–1752.
Danna EA, Sinha P, Gilbert M, Clements VK, Pulaski BA, Ostrand-Rosenberg S . Surgical removal of primary tumor reverses tumor-induced immunosuppression despite the presence of metastatic disease. Cancer Res 2004; 64:2205–2211.
Serafini P, Borrello I, Bronte V . Myeloid suppressor cells in cancer: recruitment, phenotype, properties, and mechanisms of immune suppression. Semin Cancer Biol 2006; 16:53–65.
Hock H, Hamblen MJ, Rooke HM, et al. Intrinsic requirement for zinc finger transcription factor Gfi-1 in neutrophil differentiation. Immunity 2003; 18:109–120.
Gabrilovich DI, Ostrand-Rosenberg S, Bronte V . Coordinated regulation of myeloid cells by tumours. Nat Rev Immunol 2012; 12:253–268.
Pan PY, Ma G, Weber KJ, et al. Immune stimulatory receptor CD40 is required for T-cell suppression and T regulatory cell activation mediated by myeloid-derived suppressor cells in cancer. Cancer Res 2010; 70:99–108.
Serafini P, Mgebroff S, Noonan K, Borrello I . Myeloid-derived suppressor cells promote cross-tolerance in B-cell lymphoma by expanding regulatory T cells. Cancer Res 2008; 68:5439–5449.
Hoechst B, Gamrekelashvili J, Manns MP, Greten TF, Korangy F . Plasticity of human Th17 cells and iTregs is orchestrated by different subsets of myeloid cells. Blood 2011; 117:6532–6541.
Chang LY, Lin YC, Mahalingam J, et al. Tumor-derived chemokine CCL5 enhances TGF-beta-mediated killing of CD8(+) T cells in colon cancer by T-regulatory cells. Cancer Res 2012; 72:1092–1102.
Broxmeyer HE, Sherry B, Lu L, et al. Enhancing and suppressing effects of recombinant murine macrophage inflammatory proteins on colony formation in vitro by bone marrow myeloid progenitor cells. Blood 1990; 76:1110–1116.
Broxmeyer HE, Sherry B, Cooper S, et al. Macrophage inflammatory protein (MIP)-1 beta abrogates the capacity of MIP-1 alpha to suppress myeloid progenitor cell growth. J Immunol 1991; 147:2586–2594.
Dahl R, Walsh JC, Lancki D, et al. Regulation of macrophage and neutrophil cell fates by the PU.1:C/EBPalpha ratio and granulocyte colony-stimulating factor. Nat Immunol 2003; 4:1029–1036.
Laslo P, Spooner CJ, Warmflash A, et al. Multilineage transcriptional priming and determination of alternate hematopoietic cell fates. Cell 2006; 126:755–766.
Karnoub AE, Dash AB, Vo AP, et al. Mesenchymal stem cells within tumour stroma promote breast cancer metastasis. Nature 2007; 449:557–563.
Mi Z, Bhattacharya SD, Kim VM, Guo H, Talbot LJ, Kuo PC . Osteopontin promotes CCL5-mesenchymal stromal cell-mediated breast cancer metastasis. Carcinogenesis 2011; 32:477–487.
Guy CT, Cardiff RD, Muller WJ . Induction of mammary tumors by expression of polyomavirus middle T oncogene: a transgenic mouse model for metastatic disease. Mol Cell Biol 1992; 12:954–961.
Wareing MD, Lyon AB, Lu B, Gerard C, Sarawar SR . Chemokine expression during the development and resolution of a pulmonary leukocyte response to influenza A virus infection in mice. J Leukoc Biol 2004; 76:886–895.
Tsukamoto AS, Grosschedl R, Guzman RC, Parslow T, Varmus HE . Expression of the int-1 gene in transgenic mice is associated with mammary gland hyperplasia and adenocarcinomas in male and female mice. Cell 1988; 55:619–625.
Poh TW, Bradley JM, Mukherjee P, Gendler SJ . Lack of Muc1-regulated beta-catenin stability results in aberrant expansion of CD11b+Gr1+ myeloid-derived suppressor cells from the bone marrow. Cancer Res 2009; 69:3554–3562.
Novak EK, Reddington M, Zhen L, et al. Inherited thrombocytopenia caused by reduced platelet production in mice with the gunmetal pigment gene mutation. Blood 1995; 85:1781–1789.
Karsunky H, Zeng H, Schmidt T, et al. Inflammatory reactions and severe neutropenia in mice lacking the transcriptional repressor Gfi1. Nat Genet 2002; 30:295–300.
Acknowledgements
This work was supported by a Susan G Komen Breast Cancer Foundation award (KG091243) to X M, and NIH grant (CA124820) to Y L.
Author information
Authors and Affiliations
Corresponding author
Additional information
( Supplementary information is linked to the online version of the paper on the Cell Research website.)
Supplementary information
Supplementary information, Figure S1
Efficiency of knock-down of CCL5 in 4T1 stable cell lines. (PDF 189 kb)
Supplementary information, Figure S2
Confirmation of bone marrow chimerism. (PDF 100 kb)
Supplementary information, Figure S3
Lung metastasis of bone marrow chimerism. (PDF 65 kb)
Supplementary information, Figure S4
Quantification of TAMs in tumor-bearing mice. (PDF 49 kb)
Supplementary information, Figure S5
Isolation of MO- and PMN-MDSCs. (PDF 196 kb)
Supplementary information, Figure S6
Quantification of CD31 expression in MDSC subsets. (PDF 40 kb)
Supplementary information, Figure S7
Production of NO and relative mRNA expression level of iNOS in different MDSC subsets. (PDF 94 kb)
Supplementary information, Figure S8
Measurement of MDSCs in early and late stages of 4T1 tumor growth. (PDF 68 kb)
Supplementary information, Figure S9
Measurement of CCL5 protein in early and late stages of 4T1 tumor growth. (PDF 24 kb)
Supplementary information, Figure S10
CD11b+ cells in spleens of DA3-tumor bearing WT and CCL5 KO mice. (PDF 153 kb)
Supplementary information, Figure S11
Bone marrow of tumor-free WT and CCL5 KO mice. (PDF 176 kb)
Supplementary information, Figure S12
Reciprocal bone marrow transplantation. (PDF 134 kb)
Supplementary information, Figure S13
In vitro cell growth of 4T1 and 4T1-GM/M. (PDF 67 kb)
Supplementary information, Table S1
Concentration of GM-CSF, M-CSF and G-CSF in murine serum (PDF 9 kb)
Supplementary information, Table S2
Concentration of GM-CSF, M-CSF and G-CSF in 4T1 stable cells (PDF 8 kb)
Supplementary information, Data S1
Materials and Methods (PDF 17 kb)
Rights and permissions
About this article
Cite this article
Zhang, Y., Lv, D., Kim, HJ. et al. A novel role of hematopoietic CCL5 in promoting triple-negative mammary tumor progression by regulating generation of myeloid-derived suppressor cells. Cell Res 23, 394–408 (2013). https://doi.org/10.1038/cr.2012.178
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/cr.2012.178
Keywords
This article is cited by
-
Periodontal inflammation recruits distant metastatic breast cancer cells by increasing myeloid-derived suppressor cells
Oncogene (2020)
-
The Infiltration of ICOS+ Cells in Nasopharyngeal Carcinoma is Beneficial for Improved Prognosis
Pathology & Oncology Research (2020)
-
CCL5-deficiency enhances intratumoral infiltration of CD8+ T cells in colorectal cancer
Cell Death & Disease (2018)
-
Effect of CCL5 expression in the recruitment of immune cells in triple negative breast cancer
Scientific Reports (2018)
-
Titanium implants and silent inflammation in jawbone—a critical interplay of dissolved titanium particles and cytokines TNF-α and RANTES/CCL5 on overall health?
EPMA Journal (2018)