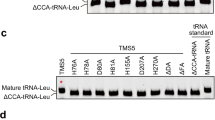
Abstract
Photoperiod- and thermo-sensitive genic male sterility (PGMS and TGMS) are the core components for hybrid breeding in crops. Hybrid rice based on the two-line system using PGMS and TGMS lines has been successfully developed and applied widely in agriculture. However, the molecular mechanism underlying the control of PGMS and TGMS remains obscure. In this study, we mapped and cloned a major locus, p/tms12-1 (photo- or thermo-sensitive genic male sterility locus on chromosome 12), which confers PGMS in the japonica rice line Nongken 58S (NK58S) and TGMS in the indica rice line Peiai 64S (PA64S, derived from NK58S). A 2.4-kb DNA fragment containing the wild-type allele P/TMS12-1 was able to restore the pollen fertility of NK58S and PA64S plants in genetic complementation. P/TMS12-1 encodes a unique noncoding RNA, which produces a 21-nucleotide small RNA that we named osa-smR5864w. A substitution of C-to-G in p/tms12-1, the only polymorphism relative to P/TMS12-1, is present in the mutant small RNA, namely osa-smR5864m. Furthermore, overexpression of a 375-bp sequence of P/TMS12-1 in transgenic NK58S and PA64S plants also produced osa-smR5864w and restored pollen fertility. The small RNA was expressed preferentially in young panicles, but its expression was not markedly affected by different day lengths or temperatures. Our results reveal that the point mutation in p/tms12-1, which probably leads to a loss-of-function for osa-smR5864m, constitutes a common cause for PGMS and TGMS in the japonica and indica lines, respectively. Our findings thus suggest that this noncoding small RNA gene is an important regulator of male development controlled by cross-talk between the genetic networks and environmental conditions.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Cheng SH, Zhuang JY, Fan YY, Du JH, Cao LY . Progress in research and development on hybrid rice: a super-domesticate in China. Ann Bot 2007; 100:959–966.
Wang Z, Zou Y, Li X, et al. Cytoplasmic male sterility of rice with boro II cytoplasm is caused by a cytotoxic peptide and is restored by two related PPR motif genes via distinct modes of mRNA silencing. Plant Cell 2006; 18:676–687.
Lin SC, Yuan LP . Hybrid rice breeding in China. In: Innovative approaches to rice breeding. Manila, Philippines: International Rice Research Institute, 1980: 35–52.
Yuan LP . Purification and production of foundation seed of rice PGMS and TGMS lines. Hybrid Rice 1994; 6:1–3.
Yang Q, Liang C, Zhuang W, et al. Characterization and identification of the candidate gene of rice thermo-sensitive genic male sterile gene tms5 by mapping. Planta 2007; 225:321–330.
Si HM, Liu WZ, Fu YP, Sun ZX, Hu GC . Current situation and suggestions for development of two-line hybrid rice in china. Chin J Rice Sci 2011; 25:544–552.
Shi MS . The discovery and study of the photosensitive recessive male-sterile rice (Oryza sativa L. subsp. japonica). Sci Agric Sin 1985; 2:44–48.
Shi MS, Deng JY . The discovery, determination and utilization of the Hubei photosensitve genic male-sterile rice (Oryza sativa subsp. japonica). Acta Agric Sin 1986; 13:107–112.
He HH, Yuan SC . Analyses of plant character in Hubei Photoperiodsensitive Genie Male-sterile Rice (HPGMR) under different light and temperature conditions. Hybrid Rice 1989; 5:42–44.
Yang SH, Cheng BY, Shen WF, Xia JH . Progress of application and breeding on two-line hybrid rice in China. Hybrid Rice 2009; 24:5–9.
Luo XH, Xia ST, Luo S, Lu ZC, Zhang ZG, Wang GY . Breeding of dual purpose genic male sterile lines and their application in two-line super rice. Hybrid Rice 2010; S1:59–63.
Luo XH, Qiu ZZ, Li RH . Pei'ai 64S, a dual purpose sterile line whose sterility is induced by low critical temperature. Hybrid Rice 1992; 1:27–29.
Lu CG, Zou JS, Hu N, Yao KM . Plant temperature for sterile alteration of a temperature-sensitive genic male sterile rice, Pei'ai64S. Sci Agric Sin 2007; 6:1283–1290.
Xu ML, Zhou GQ, Chen LB . Response of fertility of Pei'ai 64S to temperature and photoperiod in rice. Acta Agric Sin 1999; 25:772–776.
Zhang Q, Shen BZ, Dai XK, Mei MH, Saghai Maroof MA, Li ZB . Using bulked extremes and recessive class to map genes for photoperiod-sensitive genic male sterility in rice. Proc Natl Acad Sci USA 1994; 91:8675–8679.
Liu N, Shan Y, Wang FP, et al. Identification of an 85-kb DNA fragment containing pms1, a locus for photoperiod-sensitive genic male sterility in rice. Mol Genet Genomics 2001; 266:271–275.
Mei M, Chen L, Zhang Z, Li Z, Xu C, Zhang Q . Pms3 is the locus causing the original photoperiod-sensitive male sterility mutation of 'Nongken 58S'. Sci China C Life Sci 1999; 42:316–322.
Mei MH, Dai XK, Xu C, Zhang Q . Mapping and genetic analysis of the genes for photoperiod-sensitive genic male sterility in rice using the original mutant Nongken 58S. Crop Sci 1999; 39:1711–1715.
Li XH, Lu Q, Wang FL, Xu CG, Zhang QF . Separation of the two-locus inheritance of photoperiod sensitive genic male sterility in rice and precise mapping the the pms3 locus. Euphytica 2001; 119:343–348.
Lu Q, Li XH, Guo D, Xu CG, Zhang Q . Localization of pms3, a gene for photoperiod-sensitive genic male sterility, to a 28.4-kb DNA fragment. Mol Genet Genomics 2005; 273:507–511.
Peng HF, Zhang ZF, Wu B, et al. Molecular mapping of two reverse photoperiod-sensitive genic male sterility genes (rpms1 and rpms2) in rice (Oryza sativa L.). Theor Appl Genet 2008; 118:77–83.
Wang B, Xu WW, Wang JZ, et al. Tagging and mapping the thermo-sensitive genic male-sterile gene in rice (Oryza sativa L.) with molecular markers. Theor Appl Genet 1995; 91:1111–1114.
Yamagushi Y, Ikeda R, Hirasawa H, Minami M, Ujihara P . Linkage analysis of the thermo-sensitive genic male sterility gene tms2 in rice (Oryza sativa L.). Breed Sci 1997; 47:371–377.
Lopez MT, Toojinda T, Vanavichit A, Tragoonrung S . Microsatellite markers flanking the tms2 gene facilitated tropical TGMS rice line development. Crop Sci 2003; 43:2267–2271.
Subudhi PK, Borkakati RP, Virmani SS, Huang N . Molecular mapping of a thermosensitive genetic male sterility gene in rice using bulked segregant analysis. Genome 1997; 40:188–194.
Lang NT, Subudhi PK, Virmani SS, et al. Development of PCR-based markers for thermosensitive genetic male sterility gene tms3(t) in rice (Oryza Sativa L.). Hereditas 1999; 131:121–127.
Dong NV, Subudhi PK, Luong PN, et al. Molecular mapping of a rice gene conditioning thermosensitive genic male sterility using AFLP, RFLP and SSR techniques. Theor Appl Genet 2000; 100:727–734.
Wang YG, Xing QH, Deng QY, et al. Fine mapping of the rice thermo-sensitive genic male-sterile gene tms5. Theor Appl Genet 2003; 107:917–921.
Nas TM, Sanchez DL, Diaz GQ, Mendioro MS, Virmani SS . Pyramiding of thermosensitive genetic male sterility (TGMS) genes and identification of a candidate tms5 gene in rice. Euphytica 2005; 145:67–75.
Jiang D, Lu S, Zhou H, et al. Mapping of the rice (Oryza sativa L.) thermo-sensitive genic male sterile gene tms5 with EST and SSR markers. Chin Sci Bull 2006; 51:417–420.
Peng HF, Chen XH, Lu YP, et al. Fine mapping of a gene for non-pollen type thermosensitive genic male sterility in rice (Oryza sativa L.). Theor Appl Genet 2010; 120:1013–1020.
Lee DS, Chen LJ, Suh HS . Genetic characterization and fine mapping of a novel thermo-sensitive genic male-sterile gene tms6 in rice (Oryza sativa L.). Theor Appl Genet 2005; 111:1271–1277.
Liu X, Li X, Zhang X, Wang S . Genetic analysis and mapping of a thermosensitive genic male sterility gene, tms6(t), in rice (Oryza sativa L.). Genome 2010; 53:119–124.
Xu J, Wang B, Wu Y, et al. Fine mapping and candidate gene analysis of ptgms2-1, the photoperiod-thermo-sensitive genic male sterile gene in rice (Oryza sativa L.). Theor Appl Genet 2011; 122:365–372.
Zhou YF, Zhang XY, Xue QZ . Fine mapping and candidate gene prediction of the photoperiod and thermo-sensitive genic male sterile gene pms1(t) in rice. J Zhejiang Univ Sci B 2011; 12:436–447.
Jia JH, Zhang DS, Li CY, et al. Molecular mapping of the reverse thermo-sensitive genic male-sterile gene (rtms1) in rice. Theor Appl Genet 2001; 103:607–612.
Xie Z, Qi X . Diverse small RNA-directed silencing pathways in plants. Bio Bio Acta 2008; 1779:720–724.
Ramachandran V, Chen X . Small RNA metabolism in Arabidopsis. Tren Plant Sci 2008; 13:368–374.
Chen X . Small RNAs and their roles in plant development. Annu Rev Cell Dev Biol 2009; 25:21–44.
Ruiz-Ferrer V, Voinnet O . Roles of plant small RNAs in biotic stress responses. Annu Rev Plant Biol 2009; 60:485–510.
Olivier V . Origin, biogenesis, and activity of plant microRNAs. Cell 2009; 136:669–687.
Wu L, Zhang Q, Zhou H, Ni F, Wu X, Qi Y . Rice microRNA effector complexes and targets. Plant Cell 2009; 21:3421–3435.
Ji L, Liu X, Yan J, et al. ARGONAUTE10 and ARGONAUTE1 regulate the termination of floral stem cells through two microRNAs in Arabidopsis. PLoS Genet 2011; 7:e1001358. doi:10.1371/journal.pgen.1001358
Chen X . A microRNA as a translational repressor of APETALA2 in Arabidopsis flower development. Science 2004; 303:2022–2025.
Brodersen P, Sakvarelidze-Achard L, Bruun-Rasmussen M, et al. Widespread translational inhibition by plant miRNAs and siRNAs. Science 2008; 320:1185–1190.
Song X, Li P, Zhai J, et al. Roles of DCL4 and DCL3b in rice phased small RNA biogenesis. Plant J 2012; 69:462–474.
Liu B, Li P, Li X, et al. Loss of function of OsDCL1 affects microRNA accumulation and causes developmental defects in rice. Plant Physiol 2005; 139:296–305
Liu B, Chen Z, Song X, et al. Oryza sativa Dicer-like4 reveals a key role for small interfering RNA silencing in plant development. Plant Cell 2007; 19:2705–2018.
Zhu QH, Spriggs A, Matthew L, et al. A diverse set of microRNAs and microRNA-like small RNAs in developing rice grains. Genome Res 2008; 18:1456–1465.
Feng JH, Lu YG, Liu XD, Xu XB . Pollen development and its stages in rice (Oryza sativa L). Chinese J Rice Sci 2001; 15:21–28.
Zhang D, Luo X, Zhu L . Cytological analysis and genetic control of rice anther development. J Genet Genomics 2011; 38:379–390.
Yu YH, Si HM, Hu GC, Fu YP, Sun ZX . Analysis on fertility transformation reaction to photo-thermo treatments of Peiai64S from different sources. Sci Agric Sin 2006; 39:1064–1068.
Vaucheret H . Post-transcriptional small RNA pathways in plants: mechanisms and regulations. Genes Dev 2006; 20:759–771.
Liu Z, Bao W, Liang W, Yin J, Zhang D . Identification of gamyb-4 and analysis of the regulatory role of GAMYB in rice anther development. J Integ Plant Biol 2010; 52:670–678.
Kaneko M, Inukai Y, Ueguchi-Tanaka M, et al. Loss-of-function mutations of the rice GAMYB gene impair α-Amylase expression in aleurone and flower development. Plant Cell 2004; 16:33–44.
Jung KH, Han MJ, Lee YS, et al. Rice Undeveloped Tapetum1 is a major regulator of early tapetum development. Plant Cell 2005; 17:2705–2722.
Li N, Zhang DS, Liu HS, et al. The rice tapetum degeneration retardation gene is required for tapetum degradation and anther development. Plant Cell 2006; 18:2999–3014.
Li H, Pinot F, Sauveplane V, et al. Cytochrome P450 family member CYP704B2 catalyzes the ω-hydroxylation of fatty acids and is required for anther cutin biosynthesis and pollen exine formation in rice. Plant Cell 2010; 22:173–190.
Zhang D, Liang W, Yin C, Zong J, Gu F, Zhang D . OsC6, encoding a lipid transfer protein, is required for postmeiotic anther development in rice. Plant Physiol 2010; 154:149–162.
Hu L, Liang W, Yin C, et al. Rice MADS3 regulates ROS homeostasis during late anther development. Plant Cell 2011; 23:515–533.
Zhang H, Liang W, Yang X, et al. Carbon starved anther encodes a MYB domain protein that regulates sugar partitioning required for rice pollen development. Plant Cell 2010; 22:672–689.
Li X, Gao X, Wei Y, et al. Rice APOPTOSIS INHIBITOR5 coupled with two DEAD-Box adenosine 5′-triphosphate-dependent RNA helicases regulates tapetum degeneration. Plant Cell 2011; 23:1416–1434.
Nonomuraa K-I, Morohoshia A, Nakanoa M, et al. A germ cell–specific gene of the ARGONAUTE family is essential for the progression of premeiotic mitosis and meiosis during sporogenesis in rice. Plant Cell 2007; 19:2583–2594.
Dalmay T, Hamilton A, Rudd S, Angell S, Baulcombe DC . An RNA-dependent RNA polymerase gene in Arabidopsis is required for posttranscriptional gene silencing mediated by a transgene but not by a virus. Cell 2000; 101:543–553.
Li J, Jiang D, Zhou H, et al. Expression of RNA-interference/antisense transgenes by the cognate promoters of target genes is a better gene-silencing strategy to study gene functions in rice. PLoS ONE 2011; 6:e17444. doi:10.1371/annotation/6818176e-4f00-4229-a133-a83d0c3b11da
Acknowledgements
We thank Dr Ian Smith (Nara Institute of Science and Technology, Japan) for critical reading and editing of the manuscript. This work was supported by the National Basic Research Programs of China (2007CB108705 and 2011CB100203) and Genetically Modified Organisms Breeding Major Projects (2011ZX08001-004).
Author information
Authors and Affiliations
Corresponding authors
Additional information
( Supplementary information is linked to the online version of the paper on the Cell Research website.)
Supplementary information
Supplementary information, Table S1
Primers used in this study (PDF 33 kb)
Supplementary information, Figure S1
Detection of the 3′ RACE cDNA fragment of the precursor RNA in an overexpressing plant. (PDF 30 kb)
Supplementary information, Figure S2
qRT-PCR analysis of expression of nine anther development-related genes. (PDF 44 kb)
Rights and permissions
About this article
Cite this article
Zhou, H., Liu, Q., Li, J. et al. Photoperiod- and thermo-sensitive genic male sterility in rice are caused by a point mutation in a novel noncoding RNA that produces a small RNA. Cell Res 22, 649–660 (2012). https://doi.org/10.1038/cr.2012.28
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/cr.2012.28
Keywords
This article is cited by
-
LncRNA DANA1 promotes drought tolerance and histone deacetylation of drought responsive genes in Arabidopsis
EMBO Reports (2024)
-
System Analysis of Differentially Expressed miRNAs in Hexaploid Wheat Display Tissue-Specific Regulatory Role During Fe-Deficiency Response
Plant Molecular Biology Reporter (2024)
-
Establishment and Advances of Third-Generation Hybrid Rice Technology: A Review
Rice (2023)
-
Duplicate mutations of GhCYP450 lead to the production of ms5m6 male sterile line in cotton
Theoretical and Applied Genetics (2023)
-
Cotton heterosis and hybrid cultivar development
Theoretical and Applied Genetics (2023)