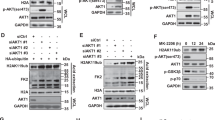
Abstract
The serine/threonine kinase Akt functions in multiple cellular processes, including cell survival and tumor development. Studies of the mechanisms that negatively regulate Akt have focused on dephosphorylation-mediated inactivation. In this study, we identified a negative regulator of Akt, MULAN, which possesses both a RING finger domain and E3 ubiquitin ligase activity. Akt was found to directly interact with MULAN and to be ubiquitinated by MULAN in vitro and in vivo. Other molecular assays demonstrated that phosphorylated Akt is a substantive target for both interaction with MULAN and ubiquitination by MULAN. The results of the functional studies suggest that the degradation of Akt by MULAN suppresses cell proliferation and viability. These data provide insight into the Akt ubiquitination signaling network.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Jones PF, Jakubowicz T, Pitossi FJ, Maurer F, Hemmings BA . Molecular cloning and identification of a serine/threonine protein kinase of the second-messenger subfamily. Proc Natl Acad Sci USA 1991; 88:4171–4175.
Burgering BM, Coffer PJ . Protein kinase B (c-Akt) in phosphatidylinositol-3-OH kinase signal transduction. Nature 1995; 376:599–602.
Franke TF . PI3K/Akt: getting it right matters. Oncogene 2008; 27:6473–6488.
Franke TF, Yang SI, Chan TO, et al. The protein kinase encoded by the Akt proto-oncogene is a target of the PDGF-activated phosphatidylinositol 3-kinase. Cell 1995; 81:727–736.
Kohn AD, Kovacina KS, Roth RA . Insulin stimulates the kinase activity of RAC-PK, a pleckstrin homology domain containing ser/thr kinase. EMBO J 1995; 14:4288–4295.
Cross DA, Alessi DR, Cohen P, Andjelkovich M, Hemmings BA . Inhibition of glycogen synthase kinase-3 by insulin mediated by protein kinase B. Nature 1995; 378:785–789.
Lustig B, Behrens J . The Wnt signaling pathway and its role in tumor development. J Cancer Res Clin Oncol 2003; 129:199–221.
Behrens J, von Kries JP, Kuhl M, et al. Functional interaction of beta-catenin with the transcription factor LEF-1. Nature 1996; 382:638–642.
Huber O, Korn R, McLaughlin J, et al. Nuclear localization of beta-catenin by interaction with transcription factor LEF-1. Mech Dev 1996; 59:3–10.
Brazil DP, Hemmings BA . Ten years of protein kinase B signalling: a hard Akt to follow. Trends Biochem Sci 2001; 26:657–664.
Luo J, Manning BD, Cantley LC . Targeting the PI3K-Akt pathway in human cancer: rationale and promise. Cancer Cell 2003; 4:257–262.
Hafizi S, Ibraimi F, Dahlback B . C1-TEN is a negative regulator of the Akt/PKB signal transduction pathway and inhibits cell survival, proliferation, and migration. FASEB J 2005; 19:971–973.
Maira SM, Galetic I, Brazil DP, et al. Carboxyl-terminal modulator protein (CTMP), a negative regulator of PKB/Akt and v-Akt at the plasma membrane. Science 2001; 294:374–380.
Du K, Herzig S, Kulkarni RN, Montminy M . TRB3: a tribbles homolog that inhibits Akt/PKB activation by insulin in liver. Science 2003; 300:1574–1577.
Paramio JM, Segrelles C, Ruiz S, Jorcano JL . Inhibition of protein kinase B (PKB) and PKCzeta mediates keratin K10-induced cell cycle arrest. Mol Cell Biol 2001; 21:7449–7459.
Mani A, Gelmann EP . The ubiquitin-proteasome pathway and its role in cancer. J Clin Oncol 2005; 23:4776–4789.
Basso AD, Solit DB, Chiosis G, et al. Akt forms an intracellular complex with heat shock protein 90 (Hsp90) and Cdc37 and is destabilized by inhibitors of Hsp90 function. J Biol Chem 2002; 277:39858–39866.
Solit DB, Basso AD, Olshen AB, Scher HI, Rosen N . Inhibition of heat shock protein 90 function down-regulates Akt kinase and sensitizes tumors to Taxol. Cancer Res 2003; 63:2139–2144.
Dickey CA, Koren J, Zhang YJ, et al. Akt and CHIP coregulate tau degradation through coordinated interactions. Proc Natl Acad Sci USA 2008; 105:3622–3627.
Suizu F, Hiramuki Y, Okumura F, et al. The E3 ligase TTC3 facilitates ubiquitination and degradation of phosphorylated Akt. Dev Cell 2009; 17:800–810.
Xiang T, Ohashi A, Huang Y, et al. Negative Regulation of AKT Activation by BRCA1. Cancer Res 2008; 68:10040–10044.
Yang WL, Wang J, Chan CH, et al. The E3 ligase TRAF6 regulates Akt ubiquitination and activation. Science 2009; 325:1134–1138.
Li W, Bengtson MH, Ulbrich A, et al. Genome-wide and functional annotation of human E3 ubiquitin ligases identifies MULAN, a mitochondrial E3 that regulates the organelle's dynamics and signaling. PLoS One 2008; 3:e1487. doi:10.1371/journal.pone.0001487
Santi SA, Lee H . The Akt isoforms are present at distinct subcellular locations. Am J Cell Physiol 2010; 298:C580–C591.
Antico Arciuch VG, Galli S, Franco MC, et al. Akt1 intramitochondrial cycling is a crucial step in the redox modulation of cell cycle progression. PloS One 2009; 4:e7523. DOI:10.1371/journal.pone.0007523
Yang JY, Yeh HY, Lin K, Wang PH . Insulin stimulates Akt translocation to mitochondria: implications on dysregulation of mitochondrial oxidative phosphorylation in diabetic myocardium. J Mol Cell Cardiol 2009; 46:919–926.
Firestone GL, Giampaolo JR, O'Keeffe BA . Stimulus-dependent regulation of serum and glucocorticoid inducible protein kinase (SGK) transcription, subcellular localization and enzymatic activity. Cell Physiol Biochem 2003; 13:1–12.
Sadowski M, Sarcevic B . Mechanisms of mono- and poly-ubiquitination: Ubiquitination specificity depends on compatibility between the E2 catalytic core and amino acid residue proximal to the lysine. Cell Div 2010; 5:19.
Alessi DR, Andjelkovic M, Caudwell B, et al. Mechanism of activation of protein kinase B by insulin and IGF-1. EMBO J 1996; 15:6541–6551.
Cohen PT, Cohen P . Discovery of a protein phosphatase activity encoded in the genome of bacteriophage lambda. Probable identity with open reading frame 221. Biochem J 1989; 260:931–934.
Zhuo S, Clemens JC, Hakes DJ, Barford D, Dixon JE . Expression, purification, crystallization, and biochemical characterization of a recombinant protein phosphatase. J Biol Chem 1993; 268:17754–17761.
Stokoe D, Stephens LR, Copeland T, et al. Dual role of phosphatidylinositol-3,4,5-trisphosphate in the activation of protein kinase B. Science 1997; 277:567–570.
Huang BX, Kim HY . Interdomain conformational changes in Akt activation revealed by chemical cross-linking and tandem mass spectrometry. Mol Cell Proteomics 2006; 5:1045–1053.
Majumder PK, Yeh JJ, George DJ, et al. Prostate intraepithelial neoplasia induced by prostate restricted Akt activation: the MPAKT model. Proc Natl Acad Sci USA 2003; 100:7841–7846.
Arcaro A, Wymann MP . Wortmannin is a potent phosphatidylinositol 3-kinase inhibitor: the role of phosphatidylinositol 3,4,5-trisphosphate in neutrophil responses. Biochem J 1993; 296(Pt 2):297–301.
Hanada M, Feng J, Hemmings BA . Structure, regulation and function of PKB/AKT – a major therapeutic target. Biochim Biophys Acta 2004; 1697:3–16.
Stephens L, Anderson K, Stokoe D, et al. Protein kinase B kinases that mediate phosphatidylinositol 3,4,5-trisphosphate-dependent activation of protein kinase B. Science 1998; 279:710–714.
Whiteman EL, Cho H, Birnbaum MJ . Role of Akt/protein kinase B in metabolism. Trends Endocrinol Metab 2002; 13:444–451.
Weston CR, Davis RJ . Signal transduction: signaling specificity – a complex affair. Science 2001; 292:2439–2440.
Meier R, Hemmings BA . Regulation of protein kinase B. J Recept Signal Transduct Res 1999; 19:121–128.
Alessi DR, Deak M, Casamayor A, et al. 3-Phosphoinositide-dependent protein kinase-1 (PDK1): structural and functional homology with the Drosophila DSTPK61 kinase. Curr Biol 1997; 7:776–789.
Riesterer O, Zingg D, Hummerjohann J, Bodis S, Pruschy M . Degradation of PKB/Akt protein by inhibition of the VEGF receptor/mTOR pathway in endothelial cells. Oncogene 2004; 23:4624–4635.
Yan D, Guo L, Wang Y . Requirement of dendritic Akt degradation by the ubiquitin-proteasome system for neuronal polarity. J Cell Biol 2006; 174:415–424.
Sasaki K, Sato M, Umezawa Y . Fluorescent indicators for Akt/protein kinase B and dynamics of Akt activity visualized in living cells. J Biol Chem 2003; 278:30945–30951.
Zhang B, Huang J, Li HL, et al. GIDE is a mitochondrial E3 ubiquitin ligase that induces apoptosis and slows growth. Cell Res 2008; 18:900–910.
Rokudai S, Fujita N, Hashimoto Y, Tsuruo T . Cleavage and inactivation of antiapoptotic Akt/PKB by caspases during apoptosis. J Cell Physiol 2000; 182:290–296.
Jope RS, Yuskaitis CJ, Beurel E . Glycogen synthase kinase-3 (GSK3): inflammation, diseases, and therapeutics. Neurochem Res 2007; 32:577–595.
Guertin DA, Sabatini DM . Defining the role of mTOR in cancer. Cancer Cell 2007; 12:9–22.
Potter CJ, Pedraza LG, Xu T . Akt regulates growth by directly phosphorylating Tsc2. Nat Cell Biol 2002; 4:658–665.
Acknowledgements
We would like to thank Dr Dirk Bohmann (University of Rochester, USA) for kindly providing the HA-Ub plasmid and Dr Zhijian Chen (University of Texas Southwestern Medical Center, USA) for the HA-Ub-K48R and HA-Ub-K63R mutant constructs. This work was supported by grants from the Ministry of Education, Science and Technology (grants 20110028646 to S An and 20100018768 to J H Lee), the National R&D Program for Cancer Control, the Ministry of Health & Welfare (0720070 to S An), and the Korea Foundation for Cancer Research (KFCR-2009-002 to S Bae) of the Republic of Korea.
Author information
Authors and Affiliations
Corresponding author
Additional information
( Supplementary information is linked to the online version of the paper on the Cell Research website)
Supplementary information
Supplementary information, Data S1
Materials and Methods (PDF 111 kb)
Supplementary information, Figure S1
The siRNA-mediated ablation of MULAN expression stabilizes endogenous Akt1 and Akt2 proteins. (PDF 138 kb)
Supplementary information, Figure S2
MULAN induced Akt1 degradation and colocalized with Akt1 in mitochondria. (PDF 3514 kb)
Supplementary information, Figure S3
Serum decreased Akt protein stability. (PDF 202 kb)
Supplementary information, Figure S4
Geldanamycin induced Akt degradation in both control cells and MULAN-depleted cells. (PDF 162 kb)
Supplementary information, Figure S5
The MULAN-mediated proteolytic degradation of Akt was rescued by wortmannin. (PDF 132 kb)
Supplementary information, Figure S6
The mutation of lysine 284 prevents the proteolytic degradation of ectopically expressed Akt by MULAN. (PDF 274 kb)
Supplementary information, Figure S7
The depletion of MULAN in HeLa cells by shRNA increased cell growth. (PDF 60 kb)
Supplementary information, Figure S8
Akt-mediated increases in cell viability are reduced by MULAN in a RING domain-dependent manner. (PDF 70 kb)
Supplementary information, Figure S9
Depletion of endogenous MULAN inhibits cell migration. (PDF 95 kb)
Supplementary information, Figure S10
MULAN-induced NF-κB activation was independent of its E3 ligase activity, and Akt-induced NF-κB activation was decreased by MULAN in an E3 ligase activity-dependent manner. (PDF 71 kb)
Supplementary information, Figure S11
Ectopically expressed Akt is degraded by MULAN in HeLa cells in the presence of the caspase inhibitor Z-VAD. (PDF 132 kb)
Supplementary information, Table S1
Primer sequences used to generate constructs for Akt and MULAN. (PDF 54 kb)
Rights and permissions
About this article
Cite this article
Bae, S., Kim, SY., Jung, J. et al. Akt is negatively regulated by the MULAN E3 ligase. Cell Res 22, 873–885 (2012). https://doi.org/10.1038/cr.2012.38
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/cr.2012.38
Keywords
This article is cited by
-
CRTAC1 enhances the chemosensitivity of non-small cell lung cancer to cisplatin by eliciting RyR-mediated calcium release and inhibiting Akt1 expression
Cell Death & Disease (2023)
-
Akt: a key transducer in cancer
Journal of Biomedical Science (2022)
-
NLRP7 deubiquitination by USP10 promotes tumor progression and tumor-associated macrophage polarization in colorectal cancer
Journal of Experimental & Clinical Cancer Research (2021)
-
Mutant ASXL1 induces age-related expansion of phenotypic hematopoietic stem cells through activation of Akt/mTOR pathway
Nature Communications (2021)
-
The role of ubiquitination and deubiquitination in cancer metabolism
Molecular Cancer (2020)