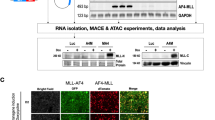
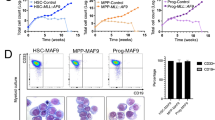
Abstract
The MLL-AF4 fusion gene is a hallmark genomic aberration in high-risk acute lymphoblastic leukemia in infants. Although it is well established that MLL-AF4 arises prenatally during human development, its effects on hematopoietic development in utero remain unexplored. We have created a human-specific cellular system to study early hemato-endothelial development in MLL-AF4-expressing human embryonic stem cells (hESCs). Functional studies, clonal analysis and gene expression profiling reveal that expression of MLL-AF4 in hESCs has a phenotypic, functional and gene expression impact. MLL-AF4 acts as a global transcriptional activator and a positive regulator of homeobox gene expression in hESCs. Functionally, MLL-AF4 enhances the specification of hemogenic precursors from hESCs but strongly impairs further hematopoietic commitment in favor of an endothelial cell fate. MLL-AF4 hESCs are transcriptionally primed to differentiate towards hemogenic precursors prone to endothelial maturation, as reflected by the marked upregulation of master genes associated to vascular-endothelial functions and early hematopoiesis. Furthermore, we report that MLL-AF4 expression is not sufficient to transform hESC-derived hematopoietic cells. This work illustrates how hESCs may provide unique insights into human development and further our understanding of how leukemic fusion genes, known to arise prenatally, regulate human embryonic hematopoietic specification.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Meyer C, Schneider B, Jakob S, et al. The MLL recombinome of acute leukemias. Leukemia 2006; 20:777–784.
Caslini C, Alarcon AS, Hess JL, Tanaka R, Murti KG, Biondi A . The amino terminus targets the mixed lineage leukemia (MLL) protein to the nucleolus, nuclear matrix and mitotic chromosomal scaffolds. Leukemia 2000; 14:1898–1908.
Pui CH . Acute lymphoblastic leukemia in children. Curr Opin Oncol 2000; 12:3–12.
Greaves MF, Maia AT, Wiemels JL, Ford AM . Leukemia in twins: lessons in natural history. Blood 2003; 102:2321–2333.
Ford AM, Ridge SA, Cabrera ME, et al. In utero rearrangements in the trithorax-related oncogene in infant leukaemias. Nature 1993; 363:358–360.
Chen W, Li Q, Hudson WA, Kumar A, Kirchhof N, Kersey JH . A murine Mll-AF4 knock-in model results in lymphoid and myeloid deregulation and hematologic malignancy. Blood 2006; 108:669–677.
Metzler M, Forster A, Pannell R, et al. A conditional model of MLL-AF4 B-cell tumourigenesis using invertor technology. Oncogene 2006; 25:3093–3103.
Krivtsov AV, Feng Z, Lemieux ME, et al. H3K79 methylation profiles define murine and human MLL-AF4 leukemias. Cancer Cell 2008; 14:355–368.
Montes R, Ayllon V, Gutierrez-Aranda I, et al. Enforced expression of MLL-AF4 fusion in cord blood CD34+ cells enhances the hematopoietic repopulating cell function and clonogenic potential but is not sufficient to initiate leukemia. Blood 2011; 117:4746–4758.
Menendez P, Bueno C, Wang L, Bhatia M . Human embryonic stem cells: potential tool for achieving immunotolerance? Stem Cell Rev 2005; 1:151–158.
Thomson JA, Itskovitz-Eldor J, Shapiro SS, et al. Embryonic stem cell lines derived from human blastocysts. Science 1998; 282:1145–1147.
Lensch MW, Daley GQ . Scientific and clinical opportunities for modeling blood disorders with embryonic stem cells. Blood 2006; 107:2605–2612.
Menendez P, Vargas A, Bueno C, et al. Quantitative analysis of bcl-2 expression in normal and leukemic human B-cell differentiation. Leukemia 2004; 18:491–498.
Ramos-Mejia V, Melen GJ, Sanchez L, et al. Nodal/Activin signaling predicts human pluripotent stem cell lines prone to differentiate towards the hematopoietic lineage. Mol Ther 2010; 18:2173–2181.
Wang L, Li L, Shojaei F, et al. Endothelial and hematopoietic cell fate of human embryonic stem cells originates from primitive endothelium with hemangioblastic properties. Immunity 2004; 21:31–41.
Diehl F, Rossig L, Zeiher AM, Dimmeler S, Urbich C . The histone methyltransferase MLL is an upstream regulator of endothelial-cell sprout formation. Blood 2007; 109:1472–1478.
Hatzipantelis ES, Athanassiou-Metaxa M, Gombakis N, et al. Thrombomodulin and von Willebrand factor: relation to endothelial dysfunction and disease outcome in children with acute lymphoblastic leukemia. Acta Haematol 2011; 125:130–135.
Bach C, Buhl S, Mueller D, Garcia-Cuellar MP, Maethner E, Slany RK . Leukemogenic transformation by HOXA cluster genes. Blood 2010; 115:2910–2918.
Stam RW, Schneider P, Hagelstein JA, et al. Gene expression profiling-based dissection of MLL translocated and MLL germline acute lymphoblastic leukemia in infants. Blood 2010; 115:2835–2844.
Tkachuk DC, Kohler S, Cleary ML . Involvement of a homolog of Drosophila trithorax by 11q23 chromosomal translocations in acute leukemias. Cell 1992; 71:691–700.
Vodyanik MA, Bork JA, Thomson JA, Slukvin II . Human embryonic stem cell-derived CD34+ cells: efficient production in the coculture with OP9 stromal cells and analysis of lymphohematopoietic potential. Blood 2005; 105:617–626.
Vodyanik MA, Thomson JA, Slukvin II . Leukosialin (CD43) defines hematopoietic progenitors in human embryonic stem cell differentiation cultures. Blood 2006; 108:2095–2105.
Lumelsky N, Blondel O, Laeng P, Velasco I, Ravin R, McKay R . Differentiation of embryonic stem cells to insulin-secreting structures similar to pancreatic islets. Science 2001; 292:1389–1394.
Pui CH, Carroll WL, Meshinchi S, Arceci RJ . Biology, risk stratification, and therapy of pediatric acute leukemias: an update. J Clin Oncol 2011; 29:551–565.
Bueno C, Montes R, Catalina P, Rodriguez R, Menendez P . Insights into the cellular origin and etiology of the infant pro-B acute lymphoblastic leukemia with MLL-AF4 rearrangement. Leukemia 2011; 25:400–410.
Bursen A, Schwabe K, Ruster B, et al. The AF4.MLL fusion protein is capable of inducing ALL in mice without requirement of MLL.AF4. Blood 2010; 115:3570–3579.
Perlingeiro RC, Kyba M, Daley GQ . Clonal analysis of differentiating embryonic stem cells reveals a hematopoietic progenitor with primitive erythroid and adult lymphoid-myeloid potential. Development 2001; 128:4597–4604.
Peters DG, Klucher KM, Perlingeiro RC, Dessain SK, Koh EY, Daley GQ . Autocrine and paracrine effects of an ES-cell derived, BCR/ABL-transformed hematopoietic cell line that induces leukemia in mice. Oncogene 2001; 20:2636–2646.
Ji J, Risueno RM, Hong S, et al. Brief report: ectopic expression of Nup98-HoxA10 augments erythroid differentiation of human embryonic stem cells. Stem Cells 2011; 29:736–741.
Wang L, Menendez P, Shojaei F, et al. Generation of hematopoietic repopulating cells from human embryonic stem cells independent of ectopic HOXB4 expression. J Exp Med 2005; 201:1603–1614.
Fang B, Zheng C, Liao L, et al. Identification of human chronic myelogenous leukemia progenitor cells with hemangioblastic characteristics. Blood 2005; 105:2733–2740.
Streubel B, Chott A, Huber D, et al. Lymphoma-specific genetic aberrations in microvascular endothelial cells in B-cell lymphomas. N Engl J Med 2004; 351:250–259.
Menendez P, Catalina P, Rodriguez R, et al. Bone marrow mesenchymal stem cells from infants with MLL-AF4+ acute leukemia harbor and express the MLL-AF4 fusion gene. J Exp Med 2009; 206:3131–3141.
Vodyanik MA, Yu J, Zhang X, et al. A mesoderm-derived precursor for mesenchymal stem and endothelial cells. Cell Stem Cell 2010; 7:718–729.
Trentin L, Giordan M, Dingermann T, Basso G, Te Kronnie G, Marschalek R . Two independent gene signatures in pediatric t(4;11) acute lymphoblastic leukemia patients. Eur J Haematol 2009; 83:406–419.
Guenther MG, Lawton LN, Rozovskaia T, et al. Aberrant chromatin at genes encoding stem cell regulators in human mixed-lineage leukemia. Genes Dev 2008; 22:3403–3408.
Bueno C, Catalina P, Melen GJ, et al. Etoposide induces MLL rearrangements and other chromosomal abnormalities in human embryonic stem cells. Carcinogenesis 2009; 30:1628–1637.
Ono R, Nakajima H, Ozaki K, et al. Dimerization of MLL fusion proteins and FLT3 activation synergize to induce multiple-lineage leukemogenesis. J Clin Invest 2005; 115:919–929.
Cortes JL, Sanchez L, Ligero G, et al. Mesenchymal stem cells facilitate the derivation of human embryonic stem cells from cryopreserved poor-quality embryos. Hum Reprod 2009; 24:1844–1851.
Montes R, Ligero G, Sanchez L, et al. Feeder-free maintenance of hESCs in mesenchymal stem cell-conditioned media: distinct requirements for TGF-beta and IGF-II. Cell Res 2009; 19:698–709.
Menendez P, Perez-Simon JA, Mateos MV, et al. Influence of the different CD34+ and CD34− cell subsets infused on clinical outcome after non-myeloablative allogeneic peripheral blood transplantation from human leucocyte antigen-identical sibling donors. Br J Haematol 2002; 119:135–143.
Gutierrez-Aranda I, Ramos-Mejia V, Bueno C, et al. Human induced pluripotent stem cells develop teratoma more efficiently and faster than human embryonic stem cells regardless the site of injection. Stem Cells 2010; 28:1568–1570.
Chadwick K, Wang L, Li L, et al. Cytokines and BMP-4 promote hematopoietic differentiation of human embryonic stem cells. Blood 2003; 102:906–915.
Menendez P, Prosper F, Bueno C, et al. Sequential analysis of CD34+ and CD34− cell subsets in peripheral blood and leukapheresis products from breast cancer patients mobilized with SCF plus G-CSF and cyclophosphamide. Leukemia 2001; 15:430–439.
Bueno C, Montes R, de la Cueva T, Gutierrez-Aranda I, Menendez P . Intra-bone marrow transplantation of human CD34(+) cells into NOD/LtSz-scid IL-2rgamma(null) mice permits multilineage engraftment without previous irradiation. Cytotherapy 2010; 12:45–49.
Bueno C, Montes R, Menendez P . The ROCK inhibitor Y-27632 negatively affects the expansion/survival of both fresh and cryopreserved cord blood-derived CD34+ hematopoietic progenitor cells. Stem Cell Rev 2010; 6:215–223.
Rubio R, Garcia-Castro J, Gutierrez-Aranda I, et al. Deficiency in p53 but not Retinoblastoma induces the transformation of mesenchymal stem cells in vitro and initiates Leiomyosarcoma in vivo. Cancer Res 2010; 70:4185–4194.
Acknowledgements
This work was funded by the CICE/FEDER (P08-CTS-3678) de la Junta de Andalucía to PM, the FIS/FEDER (PI10/00449) to PM, (PI11/00119) to CB and (PS09/02454) to MFF, the ISCIII (CP07/00059) to CB, (CP09/0063) to PJR and (EMER07/055 and PI10/00883) to JC R-M, the MICINN (PLE-2009-0111) to PM, the Foundation “Spanish Association Against Cancer”/Junta Provincial de Albacete (CI110023) to PM, the Marie Curie IIF (PIIF-GA-2009-236430) to V R-M, the CSIC (200820I172) to MFF, and the Community of Asturias (FICYT IB09-106) to MFF. AFF is supported by the IUOPA-Obra Social Cajastur.
Author information
Authors and Affiliations
Corresponding authors
Additional information
( Supplementary information is linked to the online version of the paper on the Cell Research website.)
Supplementary information
Supplementary information, Figure S1
Enforced expression of MLL-AF4 is compatible with hESC pluripotency. (PDF 138 kb)
Supplementary information, Figure S2
Q-RT-PCR comparing the expression levels of MLL-AF4 in MLL-AF4-carrying leukemic cell lines and MLL-AF4-transduced human stem cells. (PDF 37 kb)
Supplementary information, Figure S3
Cell cycle analysis reveals that the expression of MLL-AF4 promotes specification of hESCs into hemogenic precursors rather than enhanced proliferation or survival of the emerging hemogenic precursors. (PDF 62 kb)
Supplementary information, Figure S4
Spontaneous hEB differentiation reveals that MLL-AF4 augments hemogenic precursor specification and impairs further hematopoietic commitment regardless the presence of hematopoietic cytokines. (PDF 25 kb)
Supplementary information, Figure S5
Comparative analysis of our gene expression profiling (GEP) with the GEP reported by Trentin et al. (2009) and Stam et al. (2010). (PDF 100 kb)
Supplementary information, Figure S6
mRNA expression of FLT3 (A-C) and wild-type MLL (D-F) in NEO- and MLL-AF4-expressing hESCs and day 15 hEBs. (PDF 62 kb)
Supplementary information, Table S1
Annotations and analysis of the differentially regulated genes between NEO and MLL-AF4 hESCs classified by the IPA software in relevant gene functions and canonical pathways. (XLS 684 kb)
Supplementary information, Table S2
Primer sequences used in the present study (PDF 12 kb)
Rights and permissions
About this article
Cite this article
Bueno, C., Montes, R., Melen, G. et al. A human ESC model for MLL-AF4 leukemic fusion gene reveals an impaired early hematopoietic-endothelial specification. Cell Res 22, 986–1002 (2012). https://doi.org/10.1038/cr.2012.4
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/cr.2012.4
Keywords
This article is cited by
-
Panobinostat (LBH589) increase survival in adult xenografic model of acute lymphoblastic leukemia with t(4;11) but promotes antagonistic effects in combination with MTX and 6MP
Medical Oncology (2022)
-
Inhibition of DOT1L and PRMT5 promote synergistic anti-tumor activity in a human MLL leukemia model induced by CRISPR/Cas9
Oncogene (2019)
-
Molecular processes involved in B cell acute lymphoblastic leukaemia
Cellular and Molecular Life Sciences (2018)
-
NG2 antigen is involved in leukemia invasiveness and central nervous system infiltration in MLL-rearranged infant B-ALL
Leukemia (2018)
-
Reprogramming human B cells into induced pluripotent stem cells and its enhancement by C/EBPα
Leukemia (2016)