Abstract
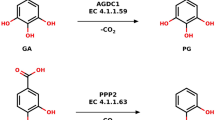
DNA methylation and demethylation regulate many crucial biological processes in mammals and are linked to many diseases. Active DNA demethylation is believed to be catalyzed by TET proteins and a putative DNA decarboxylase that may share some similarities in sequence, structure and catalytic mechanism with isoorotate decarboxylase (IDCase) that catalyzes decarboxylation of 5caU to U in fungi. We report here the structures of wild-type and mutant IDCases from Cordyceps militaris and Metarhizium anisopliae in apo form or in complexes with 5caU, U, and an inhibitor 5-nitro-uracil. IDCases adopt a typical (β/α)8 barrel fold of the amidohydrolase superfamily and function as dimers. A Zn2+ is bound at the active site and coordinated by four strictly conserved residues, one Asp and three His. The substrate is recognized by several strictly conserved residues. The functional roles of the key residues at the active site are validated by mutagenesis and biochemical studies. Based on the structural and biochemical data, we present for the first time a novel catalytic mechanism of decarboxylation for IDCases, which might also apply to other members of the amidohydrolase superfamily. In addition, our biochemical data show that IDCases can catalyze decarboxylation of 5caC to C albeit with weak activity, which is the first in vitro evidence for direct decarboxylation of 5caC to C by an enzyme. These findings are valuable in the identification of potential DNA decarboxylase in mammals.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
Abbreviations
- IDCase:
-
isoorotate decarboxylase
- T7H:
-
thymine-7-hydroxylase
- ACMSD:
-
2-amino-3-carboxymuconate-6-semialdhyde decarboxylase
- 5caU:
-
5-carboxyl-uracil (isoorotate)
- 5hmU:
-
5-hydroxymethyl-uracil
- 5fU:
-
5-formyl-uracil
- 5niU:
-
5-nitro-uracil
- 2-thioIOA:
-
5-carboxyl-2-thio-uracil
- 5mC:
-
5-methyl-cytosine
- 5hmC:
-
5-hydroxymethyl-cytosine
- 5fC:
-
5-formyl-cytosine
- 5caC:
-
5-carboxyl-cytosine
- RMSD:
-
root-mean-square deviation
References
Chow JC, Yen Z, Ziesche SM, Brown CJ . Silencing of the mammalian X chromosome. Annu Rev Genom Hum Genet 2005; 6:69–92.
Morison IM, Ramsay JP, Spencer HG . A census of mammalian imprinting. Trends Genet 2005; 21:457–465.
Miranda TB, Jones PA . DNA methylation: the nuts and bolts of repression. J Cell Physiol 2007; 213:384–390.
Suzuki MM, Bird A . DNA methylation landscapes: provocative insights from epigenomics. Nat Rev Genet 2008; 9:465–476.
Robertson KD . DNA methylation and human disease. Nat Rev Genet 2005; 6:597–610.
Gal-Yam EN, Saito Y, Egger G, Jones PA . Cancer epigenetics: modifications, screening, and therapy. Annu Rev Med 2008; 59:267–280.
Ooi SK, Bestor TH . The colorful history of active DNA demethylation. Cell 2008; 133:1145–1148.
Wu SC, Zhang Y . Active DNA demethylation: many roads lead to Rome. Nat Rev Mol Cell Biol 2010; 11:607–620.
Tahiliani M, Koh KP, Shen Y, et al. Conversion of 5-methylcytosine to 5-hydroxymethylcytosine in mammalian DNA by MLL partner TET1. Science 2009; 324:930–935.
Ito S, D'Alessio AC, Taranova OV, Hong K, Sowers LC, Zhang Y . Role of Tet proteins in 5mC to 5hmC conversion, ES-cell self-renewal and inner cell mass specification. Nature 2010; 466:1129–1133.
He YF, Li BZ, Li Z, et al. Tet-mediated formation of 5-carboxylcytosine and its excision by TDG in mammalian DNA. Science 2011; 333:1303–1307.
Ito S, Shen L, Dai Q, et al. Tet proteins can convert 5-methylcytosine to 5-formylcytosine and 5-carboxylcytosine. Science 2011; 333:1300–1303.
Pfaffeneder T, Hackner B, Truss M, et al. The discovery of 5-formylcytosine in embryonic stem cell DNA. Angew Chem Int Ed Engl 2011; 50:7008–7012.
Zhang L, Lu X, Lu J, et al. Thymine DNA glycosylase specifically recognizes 5-carboxylcytosine-modified DNA. Nat Chem Biol 2012; 8:328–330.
Schiesser S, Hackner B, Pfaffeneder T, et al. Mechanism and stem-cell activity of 5-carboxycytosine decarboxylation determined by isotope tracing. Angew Chem Int Ed Engl 2012; 51:6516–6520.
Jones ME . Pyrimidine nucleotide biosynthesis in animals: genes, enzymes, and regulation of UMP biosynthesis. Annu Rev Biochem 1980; 49:253–279.
Zrenner R, Stitt M, Sonnewald U, Boldt R . Pyrimidine and purine biosynthesis and degradation in plants. Annu Rev Plant Biol 2006; 57:805–836.
Carter NS, Yates P, Arendt CS, Boitz JM, Ullman B . Purine and pyrimidine metabolism in Leishmania. Adv Exp Med Biol 2008; 625:141–154.
Carreras CW, Santi DV . The catalytic mechanism and structure of thymidylate synthase. Annu Rev Biochem 1995; 64:721–762.
Costi MP, Ferrari S, Venturelli A, Calo S, Tondi D, Barlocco D . Thymidylate synthase structure, function and implication in drug discovery. Curr Med Chem 2005; 12:2241–2258.
Fink RM, Fink K . Utilization of radiocarbon from thymidine and other precursors of ribonucleic acid in Neurospora crassa. J Biol Chem 1962; 237:2289–2290.
Abbott MT, Schandl EK, Lee RF, Parker TS, Midgett RJ . Cofactor requirements of thymine 7-hydroxylase. Biochim Biophys Acta 1967; 132:525–528.
Watanabe MS, McCroskey RP, Abbott MT . The enzymatic conversion of 5-formyluracil to uracil 5-carboxylic acid. J Biol Chem 1970; 245:2023–2026.
Holme E, Lindstedt G, Lindstedt S, Tofft M . 18-O studies of the 2-ketoglutarate-dependent sequential oxygenation of thymine to 5-carboxyuracil. J Biol Chem 1971; 246:3314–3319.
Liu CK, Shaffer PM, Slaughter RS, McCroskey RP, Abbott MT . Stoichiometry of the pyrimidine deoxyribonucleoside 2′-hydroxylase reaction and of the conversions of 5-hydroxymethyluracil to 5-formyluracil and of the latter to uracil-5-carboxylic acid. Biochemistry 1972; 11:2172–2176.
Liu CK, Hsu CA, Abbott MT . Catalysis of three sequential dioxygenase reactions by thymine 7-hydroxylase. Arch Biochem Biophys 1973; 159:180–187.
Palmatier RD, McCroskey RP, Abbott MT . The enzymatic conversion of uracil 5-carboxylic acid to uracil and carbon dioxide. J Biol Chem 1970; 245:6706–6710.
Smiley JA, Angelot JM, Cannon RC, Marshall EM, Asch DK . Radioactivity-based and spectrophotometric assays for isoorotate decarboxylase: identification of the thymidine salvage pathway in lower eukaryotes. Anal Biochem 1999; 266:85–92.
Smiley JA, Kundracik M, Landfried DA, Barnes Sr VR, Axhemi AA . Genes of the thymidine salvage pathway: thymine-7-hydroxylase from a Rhodotorula glutinis cDNA library and iso-orotate decarboxylase from Neurospora crassa. Biochim Biophys Acta 2005; 1723:256–264.
Garavaglia S, Perozzi S, Galeazzi L, Raffaelli N, Rizzi M . The crystal structure of human alpha-amino-beta-carboxymuconate-epsilon-semialdehyde decarboxylase in complex with 1,3-dihydroxyacetonephosphate suggests a regulatory link between NAD synthesis and glycolysis. FEBS J 2009; 276:6615–6623.
Martynowski D, Eyobo Y, Li T, Yang K, Liu A, Zhang H . Crystal structure of alpha-amino-beta-carboxymuconate-epsilon-semialdehyde decarboxylase: insight into the active site and catalytic mechanism of a novel decarboxylation reaction. Biochemistry 2006; 45:10412–10421.
Wilson DK, Rudolph FB, Quiocho FA . Atomic structure of adenosine deaminase complexed with a transition-state analog: understanding catalysis and immunodeficiency mutations. Science 1991; 252:1278–1284.
Kinoshita T, Nakanishi I, Terasaka T, et al. Structural basis of compound recognition by adenosine deaminase. Biochemistry 2005; 44:10562–10569.
Porter DJ, Austin EA . Cytosine deaminase. The roles of divalent metal ions in catalysis. J Biol Chem 1993; 268:24005–24011.
Ireton GC, McDermott G, Black ME, Stoddard BL . The structure of Escherichia coli cytosine deaminase. J Mol Biol 2002; 315:687–697.
Seibert CM, Raushel FM . Structural and catalytic diversity within the amidohydrolase superfamily. Biochemistry 2005; 44:6383–6391.
Olsson MHM, Søndergaard CR, Rostkowski M, Jensen JH . PROPKA3: consistent treatment of internal and surface residues in empirical pKa predictions. J Chem Theory Comput 2011; 7:525–537.
Liu JQ, Kurihara T, Miyagi M, Esaki N, Soda K . Reaction mechanism of L-2-haloacid dehalogenase of Pseudomonas sp. YL. Identification of Asp10 as the active site nucleophile by 18O incorporation experiments. J Biol Chem 1995; 270:18309–18312.
Reinhardt LA, Svedruzic D, Chang CH, Cleland WW, Richards NGJ . Heavy atom isotope effects on the reaction catalyzed by the oxalate decarboxylase from Bacillus subtilis. J Am Chem Soc 2003; 125:1244–1252.
Otwinowski Z, Minor W . Processing of X-ray diffraction data collected in oscillation mode. Methods Enzymol 1997; 276:307–326.
Adams PD, Afonine PV, Bunkoczi G, et al. PHENIX: a comprehensive Python-based system for macromolecular structure solution. Acta Crystallogr D Biol Crystallogr 2010; 66:213–221.
Emsley P, Cowtan K . COOT: model-building tools for molecular graphics. Acta Crystallogr D Biol Crystallogr 2004; 60:2126–2132.
Murshudov GN, Vagin AA, Dodson EJ . Refinement of macromolecular structures by the maximum-likelihood method. Acta Crystallogr D Biol Crystallogr 1997; 53:240–255.
Laskowski RA, Macarthur MW, Moss DS, Thornton JM . PROCHECK: a program to check the stereochemical quality of protein structures. J Appl Crystallogr 1993; 26:283–291.
Winn MD, Ballard CC, Cowtan KD, et al. Overview of the CCP4 suite and current developments. Acta Crystallogr D Biol Crystallogr 2011; 67:235–242.
Krissinel E, Henrick K . Inference of macromolecular assemblies from crystalline state. J Mol Biol 2007; 372:774–797.
Acknowledgements
We thank the staff members at Shanghai Synchrotron Radiation Facility (SSRF) of China for technical supports in diffraction data collection, and other members of our groups for discussion. This work was supported by grants from the Ministry of Science and Technology of China (2011CB966301), the National Natural Science Foundation of China (31221001), and the Science and Technology Commission of Shanghai Municipality (10JC1416500).
Author information
Authors and Affiliations
Corresponding author
Additional information
( Supplementary information is linked to the online version of the paper on the Cell Research website.)
Supplementary information
Supplementary information, Figure S1
The chemistry for the conversion of 5mC to C in mammals is very similar to that for the conversion of T to U in the thymidine salvage pathway in fungi. (PDF 151 kb)
Supplementary information, Figure S2
Topology of the overall structural fold of CmIDCase. (PDF 76 kb)
Supplementary information, Figure S3
Comparison of IDCases from different fungi species. (PDF 1485 kb)
Supplementary information, Figure S4
CmIDCase and MaIDCase exist as dimers in both solution and crystal structure. (PDF 132 kb)
Supplementary information, Figure S5
Anomalous dispersion analyses of CmIDCase and MaIDCase. (PDF 54 kb)
Supplementary information, Figure S6
Structure of the active site. (PDF 1417 kb)
Supplementary information, Figure S7
Kinetic assays of the decarboxylation activities of wild-type CmIDCase and MaIDCase. (PDF 49 kb)
Supplementary information, Figure S8
Comparison of CmIDCase with members of the (α/β)8 barrel-containing amidohydrolase superfamily. (PDF 1487 kb)
Rights and permissions
About this article
Cite this article
Xu, S., Li, W., Zhu, J. et al. Crystal structures of isoorotate decarboxylases reveal a novel catalytic mechanism of 5-carboxyl-uracil decarboxylation and shed light on the search for DNA decarboxylase. Cell Res 23, 1296–1309 (2013). https://doi.org/10.1038/cr.2013.107
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/cr.2013.107
Keywords
This article is cited by
-
5-Formylcytosine to cytosine conversion by C–C bond cleavage in vivo
Nature Chemical Biology (2018)
-
New cofactor supports α,β-unsaturated acid decarboxylation via 1,3-dipolar cycloaddition
Nature (2015)