Abstract
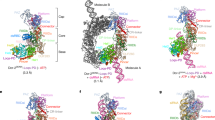
During C. elegans apoptosis, the dicer ribonuclease (DCR-1) is cleaved by the cell death protease CED-3 to generate a truncated DCR-1 (tDCR-1) with one and a half ribonuclease III (RNase III) domains, converting it into a deoxyribonuclease (DNase) that initiates apoptotic chromosome fragmentation. We performed biochemical and functional analyses to understand this unexpected RNase to DNase conversion. In full-length DCR-1, tDCR-1 DNase activity is suppressed by its N-terminal DCR-1 sequence. However, not all the sequence elements in the N-terminal DCR-1 are required for this suppression. Our deletion analysis reveals that a 20-residue α-helix sequence in DCR-1 appears to define a critical break point for the sequence required for suppressing tDCR-1 DNase activity through a structure-dependent mechanism. Removal of the N-terminal DCR-1 sequence from tDCR-1 activates a DNA-binding activity that also requires the one half RNase IIIa domain, and enables tDCR-1 to process DNA. Consistently, structural modeling of DCR-1 and tDCR-1 suggests that cleavage of DCR-1 by CED-3 may cause a conformational change that allows tDCR-1 to bind and process DNA, and may remove steric hindrance that blocks DNA access to tDCR-1. Moreover, a new DNase can be engineered using different RNase III domains, including the one from bacterial RNase III. Our results indicate that very distantly related RNase III enzymes have the potential to cleave DNA when processed proteolytically or paired with an appropriate partner that facilitates binding to DNA. We suggest the possibility that this phenomenon may be extrapolated to other ribonucleases.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Hammond SM, Bernstein E, Beach D, Hannon GJ . An RNA-directed nuclease mediates post-transcriptional gene silencing in Drosophila cells. Nature 2000; 404:293–296.
Bernstein E, Caudy AA, Hammond SM, Hannon GJ . Role for a bidentate ribonuclease in the initiation step of RNA interference. Nature 2001; 409:363–366.
Grishok A, Pasquinelli AE, Conte D, et al. Genes and mechanisms related to RNA interference regulate expression of the small temporal RNAs that control C. elegans developmental timing. Cell 2001; 106:23–34.
Hutvagner G, McLachlan J, Pasquinelli AE, Balint E, Tuschl T, Zamore PD . A cellular function for the RNA-interference enzyme dicer in the maturation of the let-7 small temporal RNA. Science 2001; 293:834–838.
Knight SW, Bass BL . A role for the RNase III enzyme DCR-1 in RNA interference and germ line development in Caenorhabditis elegans. Science 2001; 293:2269–2271.
Pham JW, Pellino JL, Lee YS, Carthew RW, Sontheimer EJ . A dicer-2-dependent 80s complex cleaves targeted mRNAs during RNAi in Drosophila. Cell 2004; 117:83–94.
Lee YS, Nakahara K, Pham JW, et al. Distinct roles for Drosophila dicer-1 and dicer-2 in the siRNA/miRNA silencing pathways. Cell 2004; 117:69–81.
Duchaine TF, Wohlschlegel JA, Kennedy S, et al. Functional proteomics reveals the biochemical niche of C. elegans DCR-1 in multiple small-RNA-mediated pathways. Cell 2006; 124:343–354.
MacRae IJ, Doudna JA . Ribonuclease revisited: structural insights into ribonuclease III family enzymes. Curr Opin Struct Biol 2007; 17:138–145.
Jaskiewicz L, Filipowicz W . Role of dicer in posttranscriptional RNA silencing. Curr Top Microbiol Immunol 2008; 320:77–97.
Nakagawa A, Shi Y, Kage-Nakadai E, Mitani S, Xue D . Caspase-dependent conversion of dicer ribonuclease into a death-promoting deoxyribonuclease. Science 2010; 328:327–334.
Zhang H, Kolb FA, Jaskiewicz L, Westhof E, Filipowicz W . Single processing center models for human dicer and bacterial RNase III. Cell 2004; 118:57–68.
MacRae IJ, Zhou K, Li F, et al. Structural basis for double-stranded RNA processing by dicer. Science 2006; 311:195–198.
Parrish JZ, Xue D . Functional genomic analysis of apoptotic DNA degradation in C. elegans. Mol Cell 2003; 11:987–996.
Parrish JZ, Xue D . Cuts can kill: the roles of apoptotic nucleases in cell death and animal development. Chromosoma 2006; 115:89–97.
Cole C, Barber JD, Barton GJ . The Jpred 3 secondary structure prediction server. Nucleic Acids Res 2008; 36:197–201.
Yang W . Nucleases: diversity of structure, function and mechanism. Q Rev Biophys 2011; 44:1–93.
Ma E, Zhou K, Kidwell MA, Doudna JA . Coordinated activities of human dicer domains in regulatory RNA processing. J Mol Biol 2012; 422:466–476.
MacRae IJ, Zhou K, Doudna JA . Structural determinants of RNA recognition and cleavage by dicer. Nat Struct Mol Biol 2007; 14:934–940.
Takeshita D, Zenno S, Lee WC, Nagata K, Saigo K, Tanokura M . Homodimeric structure and double-stranded RNA cleavage activity of the C-terminal RNase III domain of human dicer. J Mol Biol 2007; 374:106–120.
Du Z, Lee JK, Tjhen R, Stroud RM, James TL . Structural and biochemical insights into the dicing mechanism of mouse dicer: a conserved lysine is critical for dsRNA cleavage. Proc Natl Acad Sci USA 2008; 105:2391–2396.
Lehninger AL, Nelson DL, Cox MM . eds. Lehninger principles of biochemistry. 4th Edition. New York: W.H. Freeman, 2005.
Ormo M, Cubitt AB, Kallio K, Gross LA, Tsien RY, Remington SJ . Crystal structure of the Aequorea victoria green fluorescent protein. Science 1996; 273:1392–1395.
McTigue MA, Williams DR, Tainer JA . Crystal structures of a schistosomal drug and vaccine target: glutathione S-transferase from Schistosoma japonica and its complex with the leading antischistosomal drug praziquantel. J Mol Biol 1995; 246:21–27.
Brenner S . The genetics of Caenorhabditis elegans. Genetics 1974; 77:71–94.
Mello CC, Kramer JM, Stinchcomb D, Ambros V . Efficient gene transfer in C. elegans: extrachromosomal maintenance and integration of transforming sequences. EMBO J 1991; 10:3959–3970.
Gu T, Orita S, Han M . Caenorhabditis elegans SUR-5, a novel but conserved protein, negatively regulates LET-60 Ras activity during vulval induction. Mol Cell Biol 1998; 18:4556–4564.
Parrish J, Li L, Klotz K, Ledwich D, Wang X, Xue D . Mitochondrial endonuclease G is important for apoptosis in C. elegans. Nature 2001; 412:90–94.
Provost P, Dishart D, Doucet J, Frendewey D, Samuelsson B, Radmark O . Ribonuclease activity and RNA binding of recombinant human Dicer. EMBO J 2002; 21:5864–5874.
Krautz-Peterson G, Skelly PJ . Schistosoma mansoni: the dicer gene and its expression. Exp Parasitol 2008; 118:122–128.
George RA, Heringa J . An analysis of protein domain linkers: their classification and role in protein folding. Protein Eng 2002; 15:871–879.
Xue D, Shaham S, Horvitz HR . The Caenorhabditis elegans cell-death protein CED-3 is a cysteine protease with substrate specificities similar to those of the human CPP32 protease. Genes Dev 1996; 10:1073–1083.
Leaver-Fay A, Tyka M, Lewis SM, et al. ROSETTA3: an object-oriented software suite for the simulation and design of macromolecules. Methods Enzymol 2011; 487:545–574.
Xiang Z, Honig B . Extending the accuracy limits of prediction for side-chain conformations. J Mol Biol 2001; 311:421–430.
Roy A, Kucukural A, Zhang Y . I-TASSER: a unified platform for automated protein structure and function prediction. Nat Protoc 2010; 5:725–738.
Acknowledgements
We thank T Blumenthal, N Pace, and M Yarus (University of Colorado) for comments and members of the Xue lab for helpful discussions. Computational resources used in this study were supported by Tsinghua National Laboratory for Information Science and Technology. This work was supported by a Tsinghua-Peking University Life Science Center fund, the 973 Program 2013CB945602, and NIH grants R01 GM59083 and R01 GM79097.
Author information
Authors and Affiliations
Corresponding author
Additional information
( Supplementary information is linked to the online version of the paper on the Cell Research website.)
Supplementary information
Supplementary information, Figure S1
CED-3 protease cleavage assays for different DCR-1 mutants. (PDF 261 kb)
Supplementary information, Figure S2
In vitro DNase activity assays of DCR-1 proteins. (PDF 138 kb)
Supplementary information, Figure S3
DNase activity assays of DCR-1(1-1845; Δ1385-1404) and DCR-1(1-1384-GS-1405-1845). (PDF 107 kb)
Supplementary information, Figure S4
DNA binding assays. (PDF 103 kb)
Supplementary information, Figure S5
RNase activity assays of various DCR-1 truncations. (PDF 86 kb)
Supplementary information, Figure S6
Comparison of modeled DCR-1 structures with the structure of Giardia Dicer. (PDF 111 kb)
Supplementary information, Table S1
Rescue of the vulva defects of dcr-1(ok247) animals by expression of various DCR-1 truncations. (PDF 64 kb)
Rights and permissions
About this article
Cite this article
Ge, X., Zhao, X., Nakagawa, A. et al. A novel mechanism underlies caspase-dependent conversion of the dicer ribonuclease into a deoxyribonuclease during apoptosis. Cell Res 24, 218–232 (2014). https://doi.org/10.1038/cr.2013.160
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/cr.2013.160
Keywords
This article is cited by
-
Graphene quantum dots in alveolar macrophage: uptake-exocytosis, accumulation in nuclei, nuclear responses and DNA cleavage
Particle and Fibre Toxicology (2018)
-
Programmed cell death and clearance of cell corpses in Caenorhabditis elegans
Cellular and Molecular Life Sciences (2016)
-
Swiss army knives: non-canonical functions of nuclear Drosha and Dicer
Nature Reviews Molecular Cell Biology (2015)