Abstract
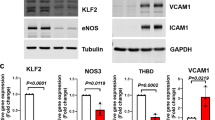
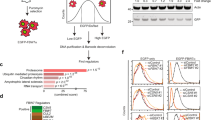
F-box and WD repeat domain-containing 7 (FBW7), the substrate-binding subunit of E3 ubiquitin ligase SCFFBW7 (a complex of SKP1, cullin-1 and FBW7), plays important roles in various physiological and pathological processes. Although FBW7 is required for vascular development, its function in the endothelium remains to be investigated. In this study, we show that FBW7 is an important regulator of endothelial functions, including angiogenesis, leukocyte adhesion and the endothelial barrier integrity. Using RNA interference, we found that the depletion of FBW7 markedly impairs angiogenesis in vitro and in vivo. We identified the zinc finger transcription factor Krüppel-like factor 2 (KLF2) as a physiological target of FBW7 in endothelial cells. Knockdown of FBW7 expression resulted in the accumulation of endogenous KLF2 protein in endothelial cells. FBW7-mediated KLF2 destruction was shown to depend on the phosphorylation of KLF2 via glycogen synthase kinase-3 (GSK3) at two conserved phosphodegrons. Mutating these phosphodegron motifs abolished the FBW7-mediated degradation and ubiquitination of KLF2. The siRNA-mediated knockdown of FBW7 showed that KLF2 is an essential target of FBW7 in the regulation of endothelial functions. Moreover, FBW7-mediated KLF2 degradation was shown to be critical for angiogenesis in teratomas and in zebrafish development. Taken together, our study suggests a role for FBW7 in the processes of endothelial cell migration, angiogenesis, inflammation and barrier integrity, and provides novel insights into the regulation of KLF2 stability in vivo.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Lee S, Chen TT, Barber CL, et al. Autocrine VEGF signaling is required for vascular homeostasis. Cell 2007; 130:691–703.
Fadini GP, Agostini C, Sartore S, Avogaro A . Endothelial progenitor cells in the natural history of atherosclerosis. Atherosclerosis 2007; 194:46–54.
Folkman J . Angiogenesis: an organizing principle for drug discovery? Nat Rev Drug Discov 2007; 6:273–286.
Rao RM, Yang L, Garcia-Cardena G, Luscinskas FW . Endothelial-dependent mechanisms of leukocyte recruitment to the vascular wall. Cir Res 2007; 101:234–247.
Sakao S, Tatsumi K, Voelkel NF . Endothelial cells and pulmonary arterial hypertension: apoptosis, proliferation, interaction and transdifferentiation. Respir Res 2009; 10:95.
McConnell BB, Yang VW . Mammalian Kruppel-like factors in health and diseases. Physiol Rev 2010; 90:1337–1381.
Nayak L, Lin Z, Jain MK . "Go with the flow": how Kruppel-like factor 2 regulates the vasoprotective effects of shear stress. Antioxid Redox Signal 2011; 15:1449–1461.
Garrido-Martin EM, Blanco FJ, Roque M, et al. Vascular injury triggers Krüppel-like factor 6 mobilization and cooperation with Sp1 to promote endothelial activation through upregulation of the activin receptor-like kinase 1 gene. Cir Res 2013; 112:113–127.
Yang DH, Hsu CF, Lin CY, Guo JY, Yu WC, Chang VH . Krüppel-like factor 10 upregulates the expression of cyclooxygenase 1 and further modulates angiogenesis in endothelial cell and platelet aggregation in gene-deficient mice. Int J Biochem Cell Biol 2012; 45:419–428.
Helbing T, Volkmar F, Goebel U, et al. Kruppel-like factor 15 regulates BMPER in endothelial cells. Cardiovasc Res 2010; 85:551–559.
Zhou G, Hamik A, Nayak L, et al. Endothelial Krüppel-like factor 4 protects against atherothrombosis in mice. J Clin Invest 2012; 122:4727–4731.
Nicoli S, Standley C, Walker P, Hurlstone A, Fogarty KE, Lawson ND . MicroRNA-mediated integration of haemodynamics and Vegf signalling during angiogenesis. Nature 2010; 464:1196–1200.
Lin Z, Natesan V, Shi H, et al. Krüppel-like factor 2 regulates endothelial barrier function. Arterioscler Thromb Vasc Biol 2010; 30:1952–1959.
Bhattacharya R, Senbanerjee S, Lin Z, et al. Inhibition of vascular permeability factor/vascular endothelial growth factor-mediated angiogenesis by the Kruppel-like factor KLF2. J Biol Chem 2005; 280:28848–28851.
SenBanerjee S, Lin Z, Atkins GB, et al. KLF2 Is a novel transcriptional regulator of endothelial proinflammatory activation. J Exp Med 2004; 199:1305–1315.
Crusio KM, King B, Reavie LB, Aifantis I . The ubiquitous nature of cancer: the role of the SCF(Fbw7) complex in development and transformation. Oncogene 2010; 29:4865–4873.
Wang W, Ha CH, Jhun BS, Wong C, Jain MK, Jin ZG . Fluid shear stress stimulates phosphorylation-dependent nuclear export of HDAC5 and mediates expression of KLF2 and eNOS. Blood 2010; 115:2971–2979.
Sen-Banerjee S, Mir S, Lin Z, et al. Krüppel-like factor 2 as a novel mediator of statin effects in endothelial cells. Circulation 2005; 112:720–726.
Wu W, Xiao H, Laguna-Fernandez A, et al. Flow-dependent regulation of Krüppel-like factor 2 is mediated by microRNA-92a. Circulation 2011; 124:633–641.
Hiroi T, Deming CB, Zhao H, et al. Proteasome inhibitors enhance endothelial thrombomodulin expression via induction of Krüppel-like transcription factors. Arterioscler Thromb Vasc Biol 2009; 29:1587–1593.
Welcker M, Clurman BE . FBW7 ubiquitin ligase: a tumour suppressor at the crossroads of cell division, growth and differentiation. Nat Rev Cancer 2008; 8:83–93.
Wang Z, Inuzuka H, Zhong J, et al. Tumor suppressor functions of FBW7 in cancer development and progression. FEBS Lett 2012; 586:1409–1418.
Liu N, Li H, Li S, et al. The Fbw7/human CDC4 tumor suppressor targets proproliferative factor KLF5 for ubiquitination and degradation through multiple phosphodegron motifs. J Biol Chem 2010; 285:18858–18867.
Wang Z, Inuzuka H, Fukushima H, et al. Emerging roles of the FBW7 tumour suppressor in stem cell differentiation. EMBO Rep 2012; 13:36–43.
Hoeck JD, Jandke A, Blake SM, et al. Fbw7 controls neural stem cell differentiation and progenitor apoptosis via Notch and c-Jun. Nat Neurosci 2010; 13:1365–1372.
Reavie L, Della Gatta G, Crusio K, et al. Regulation of hematopoietic stem cell differentiation by a single ubiquitin ligase-substrate complex. Nat Immunol 2010; 11:207–215.
Matsumoto A, Onoyama I, Sunabori T, Kageyama R, Okano H, Nakayama KI . Fbxw7-dependent degradation of Notch is required for control of “stemness” and neuronal-glial differentiation in neural stem cells. J Biol Chem 2011; 286:13754–13764.
Tetzlaff MT, Yu W, Li M, et al. Defective cardiovascular development and elevated cyclin E and Notch proteins in mice lacking the Fbw7 F-box protein. Proc Natl Acad Sci USA 2004; 101:3338–3345.
Flugel D, Gorlach A, Kietzmann T . GSK-3β regulates cell growth, migration, and angiogenesis via Fbw7 and USP28-dependent degradation of HIF-1α. Blood 2012; 119:1292–1301.
Tan M, Zhao Y, Kim SJ, et al. SAG/RBX2/ROC2 E3 ubiquitin ligase is essential for vascular and neural development by targeting NF1 for degradation. Dev Cell 2011; 21:1062–1076.
Izumi N, Helker C, Ehling M, Behrens A, Herzog W, Adams RH . Fbxw7 controls angiogenesis by regulating endothelial notch activity. PloS One 2012; 7:e41116.
Spruck CH, Strohmaier H, Sangfelt O, et al. hCDC4 gene mutations in endometrial cancer. Cancer Res 2002; 62:4535–4539.
Olsson AK, Dimberg A, Kreuger J, Claesson-Welsh L . VEGF receptor signalling - in control of vascular function. Nat Rev Mol Cell Biol 2006; 7:359–371.
Carlson CM, Endrizzi BT, Wu J, et al. Kruppel-like factor 2 regulates thymocyte and T-cell migration. Nature 2006; 442:299–302.
Williams CK, Li JL, Murga M, Harris AL, Tosato G . Up-regulation of the Notch ligand Delta-like 4 inhibits VEGF-induced endothelial cell function. Blood 2006; 107:931–939.
Welcker M, Clurman BE . Fbw7/hCDC4 dimerization regulates its substrate interactions. Cell Div 2007; 2:7.
Sako K, Fukuhara S, Minami T, et al. Angiopoietin-1 induces Krüppel-like factor 2 expression through a phosphoinositide 3-kinase/AKT-dependent activation of myocyte enhancer factor 2. J Biol Chem 2009; 284:5592–5601.
Li Z, Huang H, Boland P, et al. Embryonic stem cell tumor model reveals role of vascular endothelial receptor tyrosine phosphatase in regulating Tie2 pathway in tumor angiogenesis. Proc Natl Acad Sci USA 2009; 106:22399–22404.
Lu CW, Yabuuchi A, Chen L, Viswanathan S, Kim K, Daley GQ . Ras-MAPK signaling promotes trophectoderm formation from embryonic stem cells and mouse embryos. Nat Genet 2008; 40:921–926.
Zhang X, Srinivasan SV, Lingrel JB . WWP1-dependent ubiquitination and degradation of the lung Krüppel-like factor, KLF2. Biochem Biophys Res Commun 2004; 316:139–148.
Conkright MD, Wani MA, Lingrel JB . Lung Kruppel-like factor contains an autoinhibitory domain that regulates its transcriptional activation by binding WWP1, an E3 ubiquitin ligase. J Biol Chem 2001; 276:29299–29306.
Xie P, Tang Y, Shen S, et al. Smurf1 ubiquitin ligase targets Kruppel-like factor KLF2 for ubiquitination and degradation in human lung cancer H1299 cells. Biochem Biophys Res Commun 2011; 407:254–259.
Schulein C, Eilers M, Popov N . PI3K-dependent phosphorylation of Fbw7 modulates substrate degradation and activity. FEBS Lett 2011; 585:2151–2157.
Min SH, Lau AW, Lee TH, et al. Negative regulation of the stability and tumor suppressor function of Fbw7 by the Pin1 prolyl isomerase. Mol Cell 2012; 46:771–783.
Yokobori T, Mimori K, Iwatsuki M, et al. p53-Altered FBXW7 expression determines poor prognosis in gastric cancer cases. Cancer Res 2009; 69:3788–3794.
Mao JH, Perez-Losada J, Wu D, et al. Fbxw7/Cdc4 is a p53-dependent, haploinsufficient tumour suppressor gene. Nature 2004; 432:775–779.
Stempien-Otero A, Karsan A, Cornejo CJ, et al. Mechanisms of hypoxia-induced endothelial cell death. Role of p53 in apoptosis. J Biol Chem 1999; 274:8039–8045.
Welcker M, Orian A, Grim JE, Eisenman RN, Clurman BE . A nucleolar isoform of the Fbw7 ubiquitin ligase regulates c-Myc and cell size. Curr Biol 2004; 14:1852–1857.
Zhao D, Zheng HQ, Zhou Z, Chen C . The Fbw7 tumor suppressor targets KLF5 for ubiquitin-mediated degradation and suppresses breast cell proliferation. Cancer Res 2010; 70:4728–4738.
Bhaskaran N, van Drogen F, Ng HF, et al. Fbw7alpha and fbw7gamma collaborate to shuttle cyclin e1 into the nucleolus for multiubiquitylation. Mol Cell Biol 2013; 33:85–97.
Busino L, Millman SE, Scotto L, et al. Fbxw7α- and GSK3-mediated degradation of p100 is a pro-survival mechanism in multiple myeloma. Nat Cell Biol 2012; 14:375–385.
Biswas M, Phan D, Watanabe M, Chan JY . The Fbw7 tumor suppressor regulates nuclear factor E2-related factor 1 transcription factor turnover through proteasome-mediated proteolysis. J Biol Chem 2011; 286:39282–39289.
Tsunematsu R, Nakayama K, Oike Y, et al. Mouse Fbw7/Sel-10/Cdc4 is required for notch degradation during vascular development. J Biol Chem 2004; 279:9417–9423.
Lidington EA, Moyes DL, McCormack AM, Rose ML . A comparison of primary endothelial cells and endothelial cell lines for studies of immune interactions. Transpl Immunol 1999; 7:239–246.
Mao JH, Kim IJ, Wu D, et al. FBXW7 targets mTOR for degradation and cooperates with PTEN in tumor suppression. Science 2008; 321:1499–1502.
Vermot J, Forouhar AS, Liebling M, et al. Reversing blood flows act through klf2a to ensure normal valvulogenesis in the developing heart. PLoS Biol 2009; 7:e1000246.
Yokobori T, Mimori K, Iwatsuki M, et al. Copy number loss of FBXW7 is related to gene expression and poor prognosis in esophageal squamous cell carcinoma. Int J Oncol 2012; 41:253–259.
Fu YF, Du TT, Dong M, et al. Mir-144 selectively regulates embryonic alpha-hemoglobin synthesis during primitive erythropoiesis. Blood 2009; 113:1340–1349.
Pan W, Pham VN, Stratman AN, et al. CDP-diacylglycerol synthetase-controlled phosphoinositide availability limits VEGFA signaling and vascular morphogenesis. Blood 2012; 120:489–498.
Acknowledgements
We thank Drs Mukesh K Jain (Case Western Reserve University), Michele Pagano (New York University School of Medicine), Steven I Reed (The Scripps Research Institute), Takashi Minami (The University of Tokyo), Wenyi Wei (Harvard Medical School), Feng Liu (Institute of Zoology, Chinese Academy of Sciences), Qunying Lei (Fudan University) and Jonathan Cherry (The Johns Hopkins University) for kindly providing reagents. We thank Min Deng , Bin Wei, Gang Pei from (Institute of Biochemistry and Cell Biology, Shanghai Institutes for Biological Sciences, Chinese Academy of Sciences), and Xiaoli Zhang, Jingjing Pan, Lufan Wang and Li Lai for technical help. We also thank Drs Martin A Schwartz (Yale School of Medicine) and Hardean E Achneck (Duke University) for help with the flow chamber. We thank other members of the Wang lab for their assistance. This work was supported by the National Basic Research Program of China (973 program; 2010CB529704, 2012CB910404, and 2013CB910900), the National Natural Science Foundation of China (30800587, 30971521, 31171338 and 31222037), and the Science and Technology Commission of Shanghai Municipality (11DZ2260300). P W is a scholar of the Program for New Century Excellent Talents in University (NCET-10-0387), the Shanghai Rising-Star Program from Science and Technology Commission of Shanghai Municipality (09QA1401900), the Dawn Program of Shanghai Education Commission (11SG27), the Thousand Talents Program for Distinguished Young Scholars, and the Hundred Talents Program of the Chinese Academy of Sciences.
Author information
Authors and Affiliations
Corresponding author
Additional information
( Supplementary information is linked to the online version of the paper on the Cell Research website.)
Supplementary information
Supplementary information, Figure S1
FBW7 expression in the vascular endothelium. (PDF 412 kb)
Supplementary information, Figure S2
FBW7 shRNA inhibited the endothelial tube formation. (PDF 235 kb)
Supplementary information, Figure S3
FBW7-mediated degradation of KLF2. (PDF 287 kb)
Supplementary information, Figure S4
Interaction of KLF2 with FBW7. (PDF 225 kb)
Supplementary information, Figure S5
KLF2 contains two CPDs. (PDF 120 kb)
Supplementary information, Figure S6
Phosphorylation of KLF2 at T171 and T243. (PDF 428 kb)
Supplementary information, Figure S7
Overexpression of KLF2 had a similar effect on the angiogenesis to the FBW7 knockdown. (PDF 343 kb)
Supplementary information, Figure S8
KLF2 knockdown reduced the inhibitory effect of FBW7 on the tube formation. (PDF 245 kb)
Supplementary information, Figure S9
FBW7 knockdown in HUVECs reduced the leukocyte adhesion. (PDF 146 kb)
Supplementary information, Figure S10
FBW7 knockdown affected the cell proliferation and survival. (PDF 213 kb)
Supplementary information, Figure S11
The expression of Fbw7 mRNA in Vegfr2-positive cells at day 6 of EB formation. (PDF 149 kb)
Supplementary information, Figure S12
Knockdown of zFbw7 caused angiogenic defects in zebrafish. (PDF 475 kb)
Supplementary information, Data S1
Experimental procedures (PDF 157 kb)
Rights and permissions
About this article
Cite this article
Wang, R., Wang, Y., Liu, N. et al. FBW7 regulates endothelial functions by targeting KLF2 for ubiquitination and degradation. Cell Res 23, 803–819 (2013). https://doi.org/10.1038/cr.2013.42
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/cr.2013.42
Keywords
This article is cited by
-
Targeting the E2F1/Rb/HDAC1 axis with the small molecule HR488B effectively inhibits colorectal cancer growth
Cell Death & Disease (2023)
-
Notch activation suppresses endothelial cell migration and sprouting via miR-223-3p targeting Fbxw7
In Vitro Cellular & Developmental Biology - Animal (2022)
-
PML Suppresses Influenza Virus Replication by Promoting FBXW7 Expression
Virologica Sinica (2021)
-
Molecular characterization of sessile serrated adenoma/polyps with dysplasia/carcinoma based on immunohistochemistry, next-generation sequencing, and microsatellite instability testing: a case series study
Diagnostic Pathology (2018)
-
SCFFBW7-mediated degradation of Brg1 suppresses gastric cancer metastasis
Nature Communications (2018)