Abstract
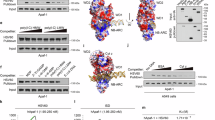
Wor1 (white-opaque switching regulator 1) is a master regulator of the white-opaque switching in Candida albicans, an opportunistic human fungal pathogen, and is associated with its pathogenicity and commensality. Wor1 contains a conserved DNA-binding region at the N-terminus, consisting of two conserved segments (WOPRa and WOPRb) connected by a non-conserved linker that can bind to specific DNA sequences of the promoter regions and then regulates the transcription. Here, we report the crystal structure of the C. albicans Wor1 WOPR segments in complex with a double-stranded DNA corresponding to one promoter region of WOR1. The sequentially separated WOPRa and WOPRb are structurally interwound together to form a compact globular domain that we term the WOPR domain. The WOPR domain represents a new conserved fungal-specific DNA-binding domain which uses primarily a conserved loop to recognize and interact specifically with a conserved 6-bp motif of the DNA in both minor and major grooves. The protein-DNA interactions are essential for WOR1 transcriptional regulation and white-to-opaque switching. The structural and biological data together reveal the molecular basis for the recognition and binding specificity of the WOPR domain with its specific DNA sequences and the function of Wor1 in the activation of transcription.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Odds FC . Candida and Candidosis, A Review and Bibliography. 2nd Edition, London: WB Saunders, 1988.
Pande K, Chen C, Noble SM . Passage through the mammalian gut triggers a phenotypic switch that promotes Candida albicans commensalism. Nat Genet 2013; 45:1088–1091.
Gow NA, van de Veerdonk FL, Brown AJ, Netea MG . Candida albicans morphogenesis and host defence: discriminating invasion from colonization. Nat Rev Microbiol 2011; 10:112–122.
Whiteway M, Bachewich C . Morphogenesis in Candida albicans. Annu Rev Microbiol 2007; 61:529–553.
Geiger J, Wessels D, Lockhart SR, Soll DR . Release of a potent polymorphonuclear leukocyte chemoattractant is regulated by white-opaque switching in Candida albicans. Infect Immun 2004; 72:667–677.
Kvaal CA, Srikantha T, Soll DR . Misexpression of the white-phase-specific gene WH11 in the opaque phase of Candida albicans affects switching and virulence. Infect Immun 1997; 65:4468–4475.
Slutsky B, Staebell M, Anderson J, Risen L, Pfaller M, Soll DR . “White-opaque transition”: a second high-frequency switching system in Candida albicans. J Bacteriol 1987; 169:189–197.
Lan CY, Newport G, Murillo LA, et al. Metabolic specialization associated with phenotypic switching in Candida albicans. Proc Natl Acad Sci USA 2002; 99:14907–14912.
Miller MG, Johnson AD . White-opaque switching in Candida albicans is controlled by mating-type locus homeodomain proteins and allows efficient mating. Cell 2002; 110:293–302.
Xie J, Tao L, Nobile CJ, et al. White-opaque switching in natural MTLa/α isolates of Candida albicans: evolutionary implications for roles in host adaptation, pathogenesis, and sex. PLoS Biol 2013; 11:e1001525.
Zordan RE, Galgoczy DJ, Johnson AD . Epigenetic properties of white-opaque switching in Candida albicans are based on a self-sustaining transcriptional feedback loop. Proc Natl Acad Sci USA 2006; 103:12807–12812.
Srikantha T, Borneman AR, Daniels KJ, et al. TOS9 regulates white-opaque switching in Candida albicans. Eukaryot Cell 2006; 5:1674–1687.
Huang G, Wang H, Chou S, Nie X, Chen J, Liu H . Bistable expression of WOR1, a master regulator of white-opaque switching in Candida albicans. Proc Natl Acad Sci USA 2006; 103:12813–12818.
Hernday AD, Lohse MB, Fordyce PM, Nobile CJ, DeRisi JL, Johnson AD . Structure of the transcriptional network controlling white-opaque switching in Candida albicans. Mol Microbiol 2013; 90:22–35.
Zordan RE, Miller MG, Galgoczy DJ, Tuch BB, Johnson AD . Interlocking transcriptional feedback loops control white-opaque switching in Candida albicans. PLoS Biol 2007; 5:e256.
Nguyen VQ, Sil A . Temperature-induced switch to the pathogenic yeast form of Histoplasma capsulatum requires Ryp1, a conserved transcriptional regulator. Proc Natl Acad Sci USA 2008; 105:4880–4885.
Michielse CB, van Wijk R, Reijnen L, et al. The nuclear protein Sge1 of Fusarium oxysporum is required for parasitic growth. PLoS Pathog 2009; 5:e1000637.
Cain CW, Lohse MB, Homann OR, Sil A, Johnson AD . A conserved transcriptional regulator governs fungal morphology in widely diverged species. Genetics 2012; 190:511–521.
Caspari T . Onset of gluconate-H+ symport in Schizosaccharomyces pombe is regulated by the kinases Wis1 and Pka1, and requires the gti1+ gene product. J Cell Sci 1997; 110 (Pt 20):2599–2608.
Michielse CB, Becker M, Heller J, Moraga J, Collado IG, Tudzynski P . The Botrytis cinerea Reg1 protein, a putative transcriptional regulator, is required for pathogenicity, conidiogenesis, and the production of secondary metabolites. Mol Plant Microbe Interact 2011; 24:1074–1085.
Okmen B, Collemare J, Griffiths S, van der Burgt A, Cox R, de Wit PJ . Functional analysis of the conserved transcriptional regulator CfWor1 in Cladosporium fulvum reveals diverse roles in the virulence of plant pathogenic fungi. Mol Microbiol 2014; 92:10–27.
Jonkers W, Dong Y, Broz K, Kistler HC . The Wor1-like protein Fgp1 regulates pathogenicity, toxin synthesis and reproduction in the phytopathogenic fungus Fusarium graminearum. PLoS Pathog 2012; 8:e1002724.
Lohse MB, Zordan RE, Cain CW, Johnson AD . Distinct class of DNA-binding domains is exemplified by a master regulator of phenotypic switching in Candida albicans. Proc Natl Acad Sci USA 2010; 107:14105–14110.
Dinkel H, Michael S, Weatheritt RJ, et al. ELM — the database of eukaryotic linear motifs. Nucleic Acids Res 2012; 40:D242–D251.
Santos MA, Tuite MF . The CUG codon is decoded in vivo as serine and not leucine in Candida albicans. Nucleic Acids Res 1995; 23:1481–1486.
Holm L, Rosenstrom P . Dali server: conservation mapping in 3D. Nucleic Acids Res 2010; 38:W545–549.
Welner DH, Lindemose S, Grossmann JG, et al. DNA binding by the plant-specific NAC transcription factors in crystal and solution: a firm link to WRKY and GCM transcription factors. Biochem J 2012; 444:395–404.
Rohs R, Jin X, West SM, Joshi R, Honig B, Mann RS . Origins of specificity in protein-DNA recognition. Annu Rev Biochem 2010; 79:233–269.
Huang G, Yi S, Sahni N, Daniels KJ, Srikantha T, Soll DR . N-acetylglucosamine induces white to opaque switching, a mating prerequisite in Candida albicans. PLoS Pathog 2010; 6:e1000806.
Otwinowski Z, Minor W . Processing of X-ray diffraction data collected in oscillation mode. In: Carter CW, Sweet RM, eds. Macromolecular Crystallography: Part A. New York: Academic Press 1997:307–326.
Adams PD, Afonine PV, Bunkoczi G, et al. PHENIX: a comprehensive Python-based system for macromolecular structure solution. Acta Crystallogr D Biol Crystallogr 2010; 66:213–221.
Murshudov GN, Vagin AA, Dodson EJ . Refinement of macromolecular structures by the maximum-likelihood method. Acta Crystallogr D Biol Crystallogr 1997; 53:240–255.
Winn MD, Ballard CC, Cowtan KD, et al. Overview of the CCP4 suite and current developments. Acta Crystallogr D Biol Crystallogr 2011; 67:235–242.
Emsley P, Cowtan K . Coot: model-building tools for molecular graphics. Acta Crystallogr D Biol Crystallogr 2004; 60:2126–2132.
Laskowski RA, Macarthur MW, Moss DS, Thornton JM . Procheck: a program to check the stereochemical quality of protein structures. J Appl Cryst 1993; 26:283–291.
Krissinel E, Henrick K . Inference of macromolecular assemblies from crystalline state. J Mol Biol 2007; 372:774–797.
Schrodinger, LLC . The PyMOL Molecular Graphics System, http://www.pymol.org, Version 1.3r1. 2010.
Lavery R, Moakher M, Maddocks JH, Petkeviciute D, Zakrzewska K . Conformational analysis of nucleic acids revisited: Curves+. Nucleic Acids Res 2009; 37:5917–5929.
Acknowledgements
We thank the staff members at BL17U of Shanghai Synchrotron Radiation Facility (SSRF), China, for technical support in diffraction data collection and other members of our groups for helpful discussion. This work was supported by the National Natural Science Foundation of China (31370105 and 31230017), the Strategic Priority Research Program of the Chinese Academy of Sciences (XDB08010300), and the Ministry of Science and Technology of China (2011CB966301 and 2010CB912103).
Author information
Authors and Affiliations
Corresponding authors
Additional information
( Supplementary information is linked to the online version of the paper on the Cell Research website.)
Supplementary information
Supplementary information, Figure S1
Sequence comparison of the WOPRb domain from different fungal species. (PDF 453 kb)
Supplementary information, Figure S2
Binding of the Wor1 WOPR domain with dsDNA of different sequences. (PDF 85 kb)
Supplementary information, Figure S3
Crystal structure of the WOPR-17bp dsDNA complex. (PDF 103 kb)
Supplementary information, Figure S4
Comparison of the WOPR-13bp dsDNA complex and the WOPR-17bp dsDNA complex. (PDF 116 kb)
Supplementary information, Figure S5
Comparison of the CaWor1 WOPR domain with the NAC domain. (PDF 144 kb)
Supplementary information, Figure S6
Sequence comparison of the WOPR domain between CaWor1 and CaPth2. (PDF 40 kb)
Supplementary information, Table S1
Interactions between theWOPR domain and the dsDNA (PDF 26 kb)
Supplementary information, Table S2
Binding affinity of WOPR mutations with dsDNA (PDF 9 kb)
Supplementary information, Table S3
dsDNA used in the EMSA (PDF 13 kb)
Supplementary information, Data S1
Materials and Methods (PDF 19 kb)
Rights and permissions
About this article
Cite this article
Zhang, S., Zhang, T., Yan, M. et al. Crystal structure of the WOPR-DNA complex and implications for Wor1 function in white-opaque switching of Candida albicans. Cell Res 24, 1108–1120 (2014). https://doi.org/10.1038/cr.2014.102
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/cr.2014.102