Abstract
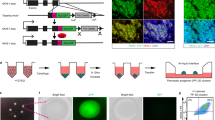
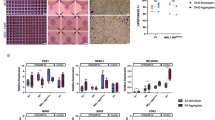
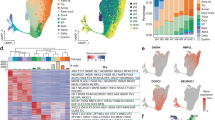
The applications of human pluripotent stem cell (hPSC)-derived cells in regenerative medicine has encountered a long-standing challenge: how can we efficiently obtain mature cell types from hPSCs? Attempts to address this problem are hindered by the complexity of controlling cell fate commitment and the lack of sufficient developmental knowledge for guiding hPSC differentiation. Here, we developed a systematic strategy to study hPSC differentiation by labeling sequential developmental genes to encompass the major developmental stages, using the directed differentiation of pancreatic β cells from hPSCs as a model. We therefore generated a large panel of pancreas-specific mono- and dual-reporter cell lines. With this unique platform, we visualized the kinetics of the entire differentiation process in real time for the first time by monitoring the expression dynamics of the reporter genes, identified desired cell populations at each differentiation stage and demonstrated the ability to isolate these cell populations for further characterization. We further revealed the expression profiles of isolated NGN3-eGFP+ cells by RNA sequencing and identified sushi domain-containing 2 (SUSD2) as a novel surface protein that enriches for pancreatic endocrine progenitors and early endocrine cells both in human embryonic stem cells (hESC)-derived pancreatic cells and in the developing human pancreas. Moreover, we captured a series of cell fate transition events in real time, identified multiple cell subpopulations and unveiled their distinct gene expression profiles, among heterogeneous progenitors for the first time using our dual reporter hESC lines. The exploration of this platform and our new findings will pave the way to obtain mature β cells in vitro.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
Accession codes
References
Cohen DE, Melton D . Turning straw into gold: directing cell fate for regenerative medicine. Nat Rev Genet 2011; 12:243–252.
Murry CE, Keller G . Differentiation of embryonic stem cells to clinically relevant populations: lessons from embryonic development. Cell 2008; 132:661–680.
Nostro MC, Keller G . Generation of β cells from human pluripotent stem cells: potential for regenerative medicine. Semin Cell Dev Biol 2012; 23:701–710.
Nostro MC, Sarangi F, Ogawa S, et al. Stage-specific signaling through TGFβ family members and WNT regulates patterning and pancreatic specification of human pluripotent stem cells. Development 2011; 138:861–871.
Nazareth EJ, Ostblom JE, Lucker PB, et al. High-throughput fingerprinting of human pluripotent stem cell fate responses and lineage bias. Nat Methods 2013; 10:1225–1231.
Rodriguez-Segui S, Akerman I, Ferrer J . GATA believe it: new essential regulators of pancreas development. J Clin Invest 2012; 122:3469–3471.
Pan FC, Wright C . Pancreas organogenesis: from bud to plexus to gland. Dev Dyn 2011; 240:530–565.
McKnight KD, Wang P, Kim SK . Deconstructing pancreas development to reconstruct human islets from pluripotent stem cells. Cell Stem Cell 2010; 6:300–308.
Bu L, Jiang X, Martin-Puig S, et al. Human ISL1 heart progenitors generate diverse multipotent cardiovascular cell lineages. Nature 2009; 460:113–117.
Wang P, Rodriguez RT, Wang J, Ghodasara A, Kim SK . Targeting SOX17 in human embryonic stem cells creates unique strategies for isolating and analyzing developing endoderm. Cell Stem Cell 2011; 8:335–346.
Micallef SJ, Li X, Schiesser JV, et al. INS(GFP/w) human embryonic stem cells facilitate isolation of in vitro derived insulin-producing cells. Diabetologia 2012; 55:694–706.
Giudice A, Trounson A . Genetic modification of human embryonic stem cells for derivation of target cells. Cell Stem Cell 2008; 2:422–433.
Kim Y, Kweon J, Kim A, et al. A library of TAL effector nucleases spanning the human genome. Nat Biotechnol 2013; 31:251–258.
Wang H, Hu YC, Markoulaki S, et al. TALEN-mediated editing of the mouse Y chromosome. Nat Biotechnol 2013; 31:530–532.
Shalem O, Sanjana NE, Hartenian E, et al. Genome-scale CRISPR-Cas9 knockout screening in human cells. Science 2014; 343:84–87.
Wang T, Wei JJ, Sabatini DM, Lander ES . Genetic screens in human cells using the CRISPR-Cas9 system. Science 2014; 343:80–84.
Pagliuca FW, Melton DA . How to make a functional β-cell. Development 2013; 140:2472–2483.
Shi Y, Hou L, Tang F, et al. Inducing embryonic stem cells to differentiate into pancreatic β cells by a novel three-step approach with activin A and all-trans retinoic acid. Stem Cells 2005; 23:656–662.
D'Amour KA, Bang AG, Eliazer S, et al. Production of pancreatic hormone-expressing endocrine cells from human embryonic stem cells. Nat Biotechnol 2006; 24:1392–1401.
Jiang J, Au M, Lu K, et al. Generation of insulin-producing islet-like clusters from human embryonic stem cells. Stem Cells 2007; 25:1940–1953.
Jiang W, Shi Y, Zhao D, et al. In vitro derivation of functional insulin-producing cells from human embryonic stem cells. Cell Res 2007; 17:333–344.
Chen S, Borowiak M, Fox JL, et al. A small molecule that directs differentiation of human ESCs into the pancreatic lineage. Nat Chem Biol 2009; 5:258–265.
Zhang D, Jiang W, Liu M, et al. Highly efficient differentiation of human ES cells and iPS cells into mature pancreatic insulin-producing cells. Cell Res 2009; 19:429–438.
Kelly OG, Chan MY, Martinson LA, et al. Cell-surface markers for the isolation of pancreatic cell types derived from human embryonic stem cells. Nat Biotechnol 2011; 29:750–756.
Rezania A, Bruin JE, Riedel MJ, et al. Maturation of human embryonic stem cell-derived pancreatic progenitors into functional islets capable of treating pre-existing diabetes in mice. Diabetes 2012; 61:2016–2029.
Zhu FF, Zhang PB, Zhang DH, et al. Generation of pancreatic insulin-producing cells from rhesus monkey induced pluripotent stem cells. Diabetologia 2011; 54:2325–2336.
Rukstalis JM, Habener JF . Neurogenin3: a master regulator of pancreatic islet differentiation and regeneration. Islets 2009; 1:177–184.
Oliver-Krasinski JM, Stoffers DA . On the origin of the β cell. Genes Dev 2008; 22:1998–2021.
Kroon E, Martinson LA, Kadoya K, et al. Pancreatic endoderm derived from human embryonic stem cells generates glucose-responsive insulin-secreting cells in vivo. Nat Biotechnol 2008; 26:443–452.
Bruin JE, Erener S, Vela J, et al. Characterization of polyhormonal insulin-producing cells derived in vitro from human embryonic stem cells. Stem Cell Res 2014; 12:194–208.
Hasegawa K, Cowan AB, Nakatsuji N, Suemori H . Efficient multicistronic expression of a transgene in human embryonic stem cells. Stem Cells 2007; 25:1707–1712.
Wang Z, Li J, Huang H, et al. An integrated chip for the high-throughput synthesis of transcription activator-like effectors. Angew Chem Int Ed Engl 2012; 51:8505–8508.
Gittes GK . Developmental biology of the pancreas: a comprehensive review. Dev Biol 2009; 326:4–35.
Zorn AM, Wells JM . Vertebrate endoderm development and organ formation. Annu Rev Cell Dev Biol 2009; 25:221–251.
Sander M, Sussel L, Conners J, et al. Homeobox gene Nkx6.1 lies downstream of Nkx2.2 in the major pathway of β-cell formation in the pancreas. Development 2000; 127:5533–5540.
Ejarque M, Cervantes S, Pujadas G, et al. Neurogenin3 cooperates with Foxa2 to autoactivate its own expression. J Biol Chem 2013; 288:11705–11717.
Schwitzgebel VM, Scheel DW, Conners JR, et al. Expression of neurogenin3 reveals an islet cell precursor population in the pancreas. Development 2000; 127:3533–3542.
Wilson ME, Scheel D, German MS . Gene expression cascades in pancreatic development. Mech Dev 2003; 120:65–80.
Collombat P, Mansouri A, Hecksher-Sorensen J, et al. Opposing actions of Arx and Pax4 in endocrine pancreas development. Genes Dev 2003; 17:2591–2603.
Rezania A, Riedel MJ, Wideman RD, et al. Production of functional glucagon-secreting α-cells from human embryonic stem cells. Diabetes 2011; 60:239–247.
Xie R, Everett LJ, Lim HW, et al. Dynamic chromatin remodeling mediated by polycomb proteins orchestrates pancreatic differentiation of human embryonic stem cells. Cell Stem Cell 2013; 12:224–237.
Desgraz R, Herrera PL . Pancreatic neurogenin 3-expressing cells are unipotent islet precursors. Development 2009; 136:3567–3574.
Watson AP, Evans RL, Egland KA . Multiple functions of sushi domain containing 2 (SUSD2) in breast tumorigenesis. Mol Cancer Res 2013; 11:74–85.
Gouzi M, Kim YH, Katsumoto K, Johansson K, Grapin-Botton A . Neurogenin3 initiates stepwise delamination of differentiating endocrine cells during pancreas development. Dev Dyn 2011; 240:589–604.
He S, Kim I, Lim MS, Morrison SJ . Sox17 expression confers self-renewal potential and fetal stem cell characteristics upon adult hematopoietic progenitors. Genes Dev 2011; 25:1613–1627.
Arntfield ME, van der Kooy D . β-Cell evolution: How the pancreas borrowed from the brain: The shared toolbox of genes expressed by neural and pancreatic endocrine cells may reflect their evolutionary relationship. Bioessays 2011; 33:582–587.
Acknowledgements
We thank Chenyan Wang, Yang Zhao, Yan Shi and Jun Xu for critical reading of the manuscript and for discussion in the preparation of this manuscript. We also thank Xiaobao Wang for conducting molecular cloning; Weichao Du, Bingqing Xie and Zijian Li for cell culture; Yinan Liu for the RT-qPCR analysis; Li Su, Shiliang Ma, Liying Du and National Center for Protein Sciences at Peking University (including but not limited to Zhonglin Fu; Zailing Bai and Jingshu Wang) for FACS; Hongxia Lv and Xiaochen Li for confocal microscopy; and Junhua Zou for G-banding analysis. This work was supported by the National Basic Research Program of China (973 program; 2012CB966401), the Key New Drug Creation and Manufacturing Program (2011ZX09102-010-03), the Ministry of Science and Technology (2013DFG30680), the National Natural Science Foundation of China (30830061), the Ministry of Education of China (111 project), and National Science and Technology Major Projects Supporting Program from Shenzhen (GJHS20120820102148947).
Author information
Authors and Affiliations
Corresponding authors
Additional information
( Supplementary information is linked to the online version of the paper on the Cell Research website.)
Supplementary information
Supplementary information, Figure S1
Targeting of eGFP or Tdtm into a series of pancreatic gene loci in hESCs. (PDF 509 kb)
Supplementary information, Figure S2
The expression of fluorescent proteins during the differentiation of reporter hESC lines into beta cells. (PDF 778 kb)
Supplementary information, Figure S3
Characterizing the different cell populations marked by individual gene reporters. (PDF 769 kb)
Supplementary information, Figure S4
Gene ontology (GO) analysis of the differentially expressed transmembrane proteins and the characterization of NGN3-eGFP-enriched and SUSD2-enriched cells in vitro. (PDF 685 kb)
Supplementary information, Figure S5
Analysis of engraftments generated by hESC-derived SUSD2-enriched cells. (PDF 367 kb)
Supplementary information, Figure S6
Characterization of SUSD2-expressing cells in vivo. (PDF 387 kb)
Supplementary information, Table S1
Fidelity of reporter cell lines (PDF 66 kb)
Supplementary information, Table S2
These are 1003 differently expressed potential surface proteins between NGN3-eGFP+ and NGN3-eGFP− cells. (XLS 860 kb)
Supplementary information, Table S3
Primary antibodies used in this study (PDF 69 kb)
Supplementary information, Table S4
Primers used for Q-PCR (PDF 65 kb)
Supplementary information, Table S5
Primers used for gene targeting (PDF 74 kb)
Supplementary information, Data S1
Extended Materials and Methods (PDF 161 kb)
Supplementary information, Movie S1
Real-time imaging showed a portion of the NGN3-eGFP+ cells gradually turned on the expression of Tdtm within 10 hours at stage 4, day 2 in DEUROD1-DR cell cultures. (MPEG 12498 kb)
Rights and permissions
About this article
Cite this article
Liu, H., Yang, H., Zhu, D. et al. Systematically labeling developmental stage-specific genes for the study of pancreatic β-cell differentiation from human embryonic stem cells. Cell Res 24, 1181–1200 (2014). https://doi.org/10.1038/cr.2014.118
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/cr.2014.118
Keywords
This article is cited by
-
Single-cell transcriptome analysis of NEUROG3+ cells during pancreatic endocrine differentiation with small molecules
Stem Cell Research & Therapy (2023)
-
Chemical combinations potentiate human pluripotent stem cell-derived 3D pancreatic progenitor clusters toward functional β cells
Nature Communications (2021)
-
CD82 is a marker to isolate β cell precursors from human iPS cells and plays a role for the maturation of β cells
Scientific Reports (2021)
-
Endocrine lineage biases arise in temporally distinct endocrine progenitors during pancreatic morphogenesis
Nature Communications (2018)
-
Towards Three-Dimensional Dynamic Regulation and In Situ Characterization of Single Stem Cell Phenotype Using Microfluidics
Molecular Biotechnology (2018)