Abstract
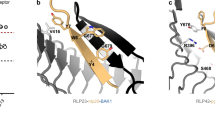
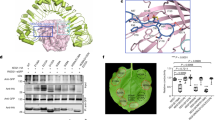
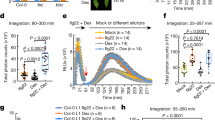
The endogenous peptides AtPep1-8 in Arabidopsis mature from the conserved C-terminal portions of their precursor proteins PROPEP1-8, respectively. The two homologous leucine-rich repeat-receptor kinases (LRR-RKs) PEPR1 and PEPR2 act as receptors of AtPeps. AtPep binding leads to stable association of PEPR1,2 with the shared receptor LRR-RK BAK1, eliciting immune responses similar to those induced by pathogens. Here we report a crystal structure of the extracellular LRR domain of PEPR1 (PEPR1LRR) in complex with AtPep1. The structure reveals that AtPep1 adopts a fully extended conformation and binds to the inner surface of the superhelical PEPR1LRR. Biochemical assays showed that AtPep1 is capable of inducing PEPR1LRR-BAK1LRR heterodimerization. The conserved C-terminal portion of AtPep1 dominates AtPep1 binding to PEPR1LRR, with the last amino acid of AtPep1 Asn23 forming extensive interactions with PEPR1LRR. Deletion of the last residue of AtPep1 significantly compromised AtPep1 interaction with PEPR1LRR. Together, our data reveal a conserved structural mechanism of AtPep1 recognition by PEPR1, providing significant insight into prediction of recognition of other peptides by their cognate LRR-RKs.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
Accession codes
References
Shiu SH, Karlowski WM, Pan R, Tzeng YH, Mayer KF, Li WH . Comparative analysis of the receptor-like kinase family in Arabidopsis and rice. Plant Cell 2004; 16:1220–1234.
Boller T, Felix G . A renaissance of elicitors: perception of microbe-associated molecular patterns and danger signals by pattern-recognition receptors. Annu Rev Plant Biol 2009; 60:379–406.
Ryan CA, Huffaker A, Yamaguchi Y . New insights into innate immunity in Arabidopsis. Cell Microbiol 2007; 9:1902–1908.
Macho AP, Zipfel C . Plant PRRs and the activation of innate immune signaling. Mol Cell 2014; 54:263–272.
Gomez-Gomez L, Boller T . FLS2: an LRR receptor-like kinase involved in the perception of the bacterial elicitor flagellin in Arabidopsis. Mol Cell 2000; 5:1003–1011.
Zipfel C, Kunze G, Chinchilla D, et al. Perception of the bacterial PAMP EF-Tu by the receptor EFR restricts Agrobacterium-mediated transformation. Cell 2006; 125:749–760.
Miya A, Albert P, Shinya T, et al. CERK1, a LysM receptor kinase, is essential for chitin elicitor signaling in Arabidopsis. Proc Natl Acad Sci USA 2007; 104:19613–19618.
Wan J, Zhang XC, Neece D, et al. A LysM receptor-like kinase plays a critical role in chitin signaling and fungal resistance in Arabidopsis. Plant Cell 2008; 20:471–481.
Chinchilla D, Zipfel C, Robatzek S, et al. A flagellin-induced complex of the receptor FLS2 and BAK1 initiates plant defence. Nature 2007; 448:497–500.
Chinchilla D, Shan L, He P, de Vries S, Kemmerling B . One for all: the receptor-associated kinase BAK1. Trends Plant Sci 2009; 14:535–541.
Li J, Wen J, Lease KA, Doke JT, Tax FE, Walker JC . BAK1, an Arabidopsis LRR receptor-like protein kinase, interacts with BRI1 and modulates brassinosteroid signaling. Cell 2002; 110:213–222.
Nam KH, Li J . BRI1/BAK1, a receptor kinase pair mediating brassinosteroid signaling. Cell 2002; 110:203–212.
Liu T, Liu Z, Song C, et al. Chitin-induced dimerization activates a plant immune receptor. Science 2012; 336:1160–1164.
Huffaker A, Pearce G, Ryan CA . An endogenous peptide signal in Arabidopsis activates components of the innate immune response. Proc Natl Acad Sci USA 2006; 103:10098–10103.
Pearce G, Yamaguchi Y, Munske G, Ryan CA . Structure-activity studies of AtPep1, a plant peptide signal involved in the innate immune response. Peptides 2008; 29:2083–2089.
Bartels S, Lori M, Mbengue M, et al. The family of Peps and their precursors in Arabidopsis: differential expression and localization but similar induction of pattern-triggered immune responses. J Exp Bot 2013; 64:5309–5321.
Ryan CA, Pearce G . Systemins: a functionally defined family of peptide signals that regulate defensive genes in Solanaceae species. Proc Natl Acad Sci USA 2003; 100 Suppl 2:14577–14580.
Yamaguchi Y, Pearce G, Ryan CA . The cell surface leucine-rich repeat receptor for AtPep1, an endogenous peptide elicitor in Arabidopsis, is functional in transgenic tobacco cells. Proc Natl Acad Sci USA 2006; 103:10104–10109.
Krol E, Mentzel T, Chinchilla D, et al. Perception of the Arabidopsis danger signal peptide 1 involves the pattern recognition receptor AtPEPR1 and its close homologue AtPEPR2. J Biol Chem 2010; 285:13471–13479.
Yamaguchi Y, Huffaker A, Bryan AC, Tax FE, Ryan CA . PEPR2 is a second receptor for the Pep1 and Pep2 peptides and contributes to defense responses in Arabidopsis. Plant Cell 2010; 22:508–522.
Qi Z, Verma R, Gehring C, et al. Ca2+ signaling by plant Arabidopsis thaliana Pep peptides depends on AtPepR1, a receptor with guanylyl cyclase activity, and cGMP-activated Ca2+ channels. Proc Natl Acad Sci USA 2010; 107:21193–21198.
Ma Y, Walker RK, Zhao Y, Berkowitz GA . Linking ligand perception by PEPR pattern recognition receptors to cytosolic Ca2+ elevation and downstream immune signaling in plants. Proc Natl Acad Sci USA 2012; 109:19852–19857.
Postel S, Kufner I, Beuter C, et al. The multifunctional leucine-rich repeat receptor kinase BAK1 is implicated in Arabidopsis development and immunity. Eur J Cell Biol 2010; 89:169–174.
Schulze B, Mentzel T, Jehle AK, et al. Rapid heteromerization and phosphorylation of ligand-activated plant transmembrane receptors and their associated kinase BAK1. J Biol Chem 2010; 285:9444–9451.
Huffaker A, Ryan CA . Endogenous peptide defense signals in Arabidopsis differentially amplify signaling for the innate immune response. Proc Natl Acad Sci USA 2007; 104:10732–10736.
Liu Z, Wu Y, Yang F, et al. BIK1 interacts with PEPRs to mediate ethylene-induced immunity. Proc Natl Acad Sci USA 2013; 110:6205–6210.
Tintor N, Ross A, Kanehara K, et al. Layered pattern receptor signaling via ethylene and endogenous elicitor peptides during Arabidopsis immunity to bacterial infection. Proc Natl Acad Sci USA 2013; 110:6211–6216.
Flury P, Klauser D, Schulze B, Boller T, Bartels S . The anticipation of danger: microbe-associated molecular pattern perception enhances AtPep-triggered oxidative burst. Plant Physiol 2013; 161:2023–2035.
Ross A, Yamada K, Hiruma K, et al. The Arabidopsis PEPR pathway couples local and systemic plant immunity. EMBO J 2014; 33:62–75.
Sun Y, Li L, Macho AP, et al. Structural basis for flg22-induced activation of the Arabidopsis FLS2-BAK1 immune complex. Science 2013; 342:624–628.
Sun Y, Han Z, Tang J, et al. Structure reveals that BAK1 as a co-receptor recognizes the BRI1-bound brassinolide. Cell Res 2013; 23:1326–1329.
Santiago J, Henzler C, Hothorn M . Molecular mechanism for plant steroid receptor activation by somatic embryogenesis co-receptor kinases. Science 2013; 341:889–892.
Wang ZY, Bai MY, Oh E, Zhu JY . Brassinosteroid signaling network and regulation of photomorphogenesis. Annu Rev Genet 2012; 46:701–724.
Hothorn M, Belkhadir Y, Dreux M, et al. Structural basis of steroid hormone perception by the receptor kinase BRI1. Nature 2011; 474:467–471.
She J, Han Z, Kim TW, et al. Structural insight into brassinosteroid perception by BRI1. Nature 2011, 474:472–476.
Kunze G, Zipfel C, Robatzek S, Niehaus K, Boller T, Felix G . The N terminus of bacterial elongation factor Tu elicits innate immunity in Arabidopsis plants. Plant Cell 2004; 16:3496–3507.
Felix G, Duran JD, Volko S, Boller T . Plants have a sensitive perception system for the most conserved domain of bacterial flagellin. Plant J 1999; 18:265–276.
Kondo T, Sawa S, Kinoshita A, et al. A plant peptide encoded by CLV3 identified by in situ MALDI-TOF MS analysis. Science 2006; 313:845–848.
Song DH, Lee JO . Sensing of microbial molecular patterns by toll-like receptors. Immunol Rev 2012; 250:216–229.
Han Z, Sun Y, Chai J . Structural insight into the activation of plant receptor kinases. Curr Opin Plant Biol 2014; 20C:55–63.
Otwinowski Z, Minor W . Processing of X-ray diffraction data collected in oscillation mode. Method Enzymol 1997; 276:307–326.
McCoy AJ, Grosse-Kunstleve RW, Adams PD, Winn MD, Storoni LC, Read RJ . Phaser crystallographic software. J Appl Crystallogr 2007; 40:658–674.
Emsley P, Cowtan K . Coot: model-building tools for molecular graphics. Acta Crystallogr D Biol Crystallogr 2004; 60:2126–2132.
Adams PD, Grosse-Kunstleve RW, Hung LW, et al. PHENIX: building new software for automated crystallographic structure determination. Acta Crystallogr D Biol Crystallogr 2002; 58:1948–1954.
Gong X, Wang P, Yang F, et al. Protein-protein docking with binding site patch prediction and network-based terms enhanced combinatorial scoring. Proteins 2010; 78:3150–3155.
Pronk S, Pall S, Schulz R, et al. GROMACS 4.5: a high-throughput and highly parallel open source molecular simulation toolkit. Bioinformatics 2013; 29:845–854.
Acknowledgements
We thank Yu F and He J at Shanghai Synchrotron Radiation Facility (SSRF). The research was funded by the National Natural Science Foundation of China (31420103906 and 31130063), the Chinese Ministry of Science and Technology (2014CB910101) and the National Outstanding Young Scholar Science Foundation of China (31025008 to JC).
Author information
Authors and Affiliations
Corresponding author
Additional information
( Supplementary information is linked to the online version of the paper on the Cell Research website.)
Supplementary information
Supplementary information, Figure S1
AtPep1,3,5,6,7,8 interact with PEPR1LRR. (PDF 197 kb)
Supplementary information, Figure S2
Chemically synthesized AtPep4,5 with their C-terminal extensions deleted but not the wild type ones induce PEPR1LRR-BAK1LRR heterodimerization (PDF 238 kb)
Supplementary information, Figure S3
Sequence alignment of the extracellular LRR domains of PEPR1 and PEPR2 from Arabidopsis. (PDF 357 kb)
Supplementary information, Table S1
Data Collection Statistics (PDF 443 kb)
Supplementary information, Data S1
SI Materials and Methods (PDF 158 kb)
Rights and permissions
About this article
Cite this article
Tang, J., Han, Z., Sun, Y. et al. Structural basis for recognition of an endogenous peptide by the plant receptor kinase PEPR1. Cell Res 25, 110–120 (2015). https://doi.org/10.1038/cr.2014.161
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/cr.2014.161
Keywords
This article is cited by
-
Rapid alkalinization factor: function, regulation, and potential applications in agriculture
Stress Biology (2023)
-
A cell-free approach to identify binding hotspots in plant immune receptors
Scientific Reports (2022)
-
Amplification of cell signaling and disease resistance by an immunity receptor Ve1Ve2 heterocomplex in plants
Communications Biology (2022)
-
Prunus persica plant endogenous peptides PpPep1 and PpPep2 cause PTI-like transcriptome reprogramming in peach and enhance resistance to Xanthomonas arboricola pv. pruni
BMC Genomics (2021)
-
Structural basis for Ca2+-dependent activation of a plant metacaspase
Nature Communications (2020)