Abstract
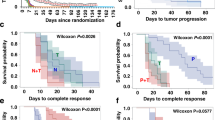
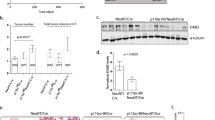
Combinatorial targeted therapies are more effective in treating cancer by blocking by-pass mechanisms or inducing synthetic lethality. However, their clinical application is hampered by resistance and toxicity. To meet this important challenge, we developed and tested a novel concept of biomarker-guided sequential applications of various targeted therapies using ErbB2-overexpressing/PTEN-low, highly aggressive breast cancer as our model. Strikingly, sustained activation of ErbB2 and downstream pathways drives trastuzumab resistance in both PTEN-low/trastuzumab-resistant breast cancers from patients and mammary tumors with intratumoral heterogeneity from genetically-engineered mice. Although lapatinib initially inhibited trastuzumab-resistant mouse tumors, tumors by-passed the inhibition by activating the PI3K/mTOR signaling network as shown by the quantitative protein arrays. Interestingly, activation of the mTOR pathway was also observed in neoadjuvant lapatinib-treated patients manifesting lapatinib resistance. Trastuzumab + lapatinib resistance was effectively overcome by sequential application of a PI3K/mTOR dual kinase inhibitor (BEZ235) with no significant toxicity. However, our p-RTK array analysis demonstrated that BEZ235 treatment led to increased ErbB2 expression and phosphorylation in genetically-engineered mouse tumors and in 3-D, but not 2-D, culture, leading to BEZ235 resistance. Mechanistically, we identified ErbB2 protein stabilization and activation as a novel mechanism of BEZ235 resistance, which was reversed by subsequent treatment with lapatinib + BEZ235 combination. Remarkably, this sequential application of targeted therapies guided by biomarker changes in the tumors rapidly evolving resistance doubled the life-span of mice bearing exceedingly aggressive tumors. This fundamentally novel approach of using targeted therapies in a sequential order can effectively target and reprogram the signaling networks in cancers evolving resistance during treatment.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Slamon D, Clark G, Wong S, Levin W, Ullrich A, McGuire W . Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science 1987; 235:177–182.
Carter P, Presta L, Gorman CM, et al. Humanization of an anti-p185HER2 antibody for human cancer therapy. Proc Natl Acad Sci USA 1992; 89:4285–4289.
Van Pelt AE, Mohsin S, Elledge RM, et al. Neoadjuvant trastuzumab and docetaxel in patients with breast cancer: preliminary results. Clin Breast Cancer 2003; 4:348–353.
Romond EH, Perez EA, Bryant J, et al. Trastuzumab plus adjuvant chemotherapy for operable her2-positive breast cancer. N Engl J Med 2005; 353:1673–1684.
Vogel CL, Cobleigh MA, Tripathy D, et al. Efficacy and safety of trastuzumab as a single agent in first-line treatment of HER2-overexpressing metastatic breast cancer. J Clin Oncol 2002; 20:719–726.
Geyer CE, Forster J, Lindquist D, et al. Lapatinib plus capecitabine for HER2-positive advanced breast cancer. N Engl J Med 2006; 355:2733–2743.
Esteva FJ, Yu D, Hung MC, Hortobagyi GN . Molecular predictors of response to trastuzumab and lapatinib in breast cancer. Nat Rev Clin Oncol 2010; 7:98–107.
Arteaga CL, Sliwkowski MX, Osborne CK, Perez EA, Puglisi F, Gianni L . Treatment of HER2-positive breast cancer: current status and future perspectives. Nat Rev Clin Oncol 2012; 9:16–32.
Nagata Y, Lan KH, Zhou X, et al. PTEN activation contributes to tumor inhibition by trastuzumab, and loss of PTEN predicts trastuzumab resistance in patients. Cancer Cell 2004; 6:117–27.
Berns K, Horlings HM, Hennessy BT, et al. A functional genetic approach identifies the PI3K pathway as a major determinant of trastuzumab resistance in breast cancer. Cancer Cell 2007; 12:395–402.
Salmena L, Carracedo A, Pandolfi PP . Tenets of PTEN tumor suppression. Cell 2008; 133:403–414.
Schade B, Rao T, Dourdin N, et al. PTEN deficiency in a luminal ErbB-2 mouse model results in dramatic acceleration of mammary tumorigenesis and metastasis. J Biol Chem 2009; 284:19018–19026.
Wang Q, Li S-H, Wang H, et al. Concomitant targeting of tumor cells and induction of T-cell response synergizes to effectively inhibit trastuzumab-resistant breast cancer. Cancer Res 2012; 72:4417–4428.
Zhang S, Huang WC, Li P, et al. Combating trastuzumab resistance by targeting SRC, a common node downstream of multiple resistance pathways. Nat Med 2011; 17:461–469.
Chakrabarty A, Bhola NE, Sutton C, et al. Trastuzumab-resistant cells rely on a HER2-PI3K-FoxO-survivin axis and are sensitive to PI3K inhibitors. Cancer Res 2013; 73:1190–1200.
Shimizu T, Tolcher AW, Papadopoulos KP, et al. The clinical effect of the dual-targeting strategy involving PI3K/AKT/mTOR and RAS/MEK/ERK pathways in patients with advanced cancer. Clin Cancer Res 2012; 18:2316–2325.
Esteva FJ, Wang J, Lin F, et al. CD40 signaling predicts response to preoperative trastuzumab and concomitant paclitaxel followed by 5-fluorouracil, epirubicin, and cyclophosphamide in HER-2-overexpressing breast cancer. Breast Cancer Res 2007; 9:R87.
Kamel D, Brady B, Tabchy A, B. Mills G, Hennessy B . Proteomic classification of breast cancer. Curr Drug Targets 2012; 13:1495–1509.
Zhang H, Wang Q, Montone KT, et al. Shared antigenic epitopes and pathobiological functions of anti-p185her2/neu monoclonal antibodies. Exp Mol Pathol 1999; 67:15–25.
Blackwell KL, Burstein HJ, Storniolo AM, et al. Overall survival benefit with lapatinib in combination with trastuzumab for patients with human epidermal growth factor receptor 2-positive metastatic breast cancer: final results from the EGF104900 Study. J Clin Oncol 2012; 30:2585–2592.
Dave B, Migliaccio I, Gutierrez MC, et al. Loss of phosphatase and tensin homolog or phosphoinositol-3 kinase activation and response to trastuzumab or lapatinib in human epidermal growth factor receptor 2-overexpressing locally advanced breast cancers. J Clin Oncol 2011; 29:166–173.
Subramanian A, Tamayo P, Mootha VK, et al. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc Natl Acad Sci USA 2005; 102:15545–15550.
Majumder PK, Febbo PG, Bikoff R, et al. mTOR inhibition reverses Akt-dependent prostate intraepithelial neoplasia through regulation of apoptotic and HIF-1-dependent pathways. Nat Med 2004; 10:594–601.
Cobleigh MA, Vogel CL, Tripathy D, et al. Multinational study of the efficacy and safety of humanized anti-HER2 monoclonal antibody in women who have HER2-overexpressing metastatic breast cancer that has progressed after chemotherapy for metastatic disease. J Clin Oncol 1999; 17:2639–2648.
Eichhorn PJA, Gili M, Scaltriti M, et al. Phosphatidylinositol 3-kinase hyperactivation results in lapatinib resistance that is reversed by the mTOR/phosphatidylinositol 3-kinase inhibitor NVP-BEZ235. Cancer Res 2008; 68:9221–9230.
Wang L, Zhang Q, Zhang J, et al. PI3K pathway activation results in low efficacy of both trastuzumab and lapatinib. BMC Cancer 2011; 11:248.
Xia W, Husain I, Liu L, et al. Lapatinib antitumor activity is not dependent upon phosphatase and tensin homologue deleted on chromosome 10 in ErbB2-overexpressing breast cancers. Cancer Res 2007; 67:1170–1175.
Jegg AM, Ward TM, Iorns E, et al. PI3K independent activation of mTORC1 as a target in lapatinib-resistant ERBB2+ breast cancer cells. Breast Cancer Res Treat 2012; 136:683–692.
Muranen T, Selfors LM, Worster DT, et al. Inhibition of PI3K/mTOR leads to adaptive resistance in matrix-attached cancer cells. Cancer Cell 2012; 21:227–239.
Lee MJ, Ye AS, Gardino AK, et al. Sequential application of anticancer drugs enhances cell death by rewiring apoptotic signaling networks. Cell 2012; 149:780–794.
Zhu J, Blenis J, Yuan J . Activation of PI3K/Akt and MAPK pathways regulates Myc-mediated transcription by phosphorylating and promoting the degradation of Mad1. Proc Natl Acad Sci USA 2008; 105:6584–6589.
Muellner MK, Uras IZ, Gapp B V, et al. A chemical-genetic screen reveals a mechanism of resistance to PI3K inhibitors in cancer. Nat Chem Biol 2011; 7:787–793.
Ilic N, Utermark T, Widlund HR, Roberts TM . PI3K-targeted therapy can be evaded by gene amplification along the MYC-eukaryotic translation initiation factor 4E (eIF4E) axis. Proc Natl Acad Sci USA 2011; 108:E699–E708.
Liu P, Cheng H, Santiago S, et al. Oncogenic PIK3CA-driven mammary tumors frequently recur via PI3K pathway-dependent and PI3K pathway-independent mechanisms. Nat Med 2011; 17:1116–1120.
Milliken EL, Lozada KL, Johnson E, et al. Ovarian hyperstimulation induces centrosome amplification and aneuploid mammary tumors independently of alterations in p53 in a transgenic mouse model of breast cancer. Oncogene 2008; 27:1759–1766.
Chen Z, Cheng K, Walton Z, et al. A murine lung cancer co-clinical trial identifies genetic modifiers of therapeutic response. Nature 2012; 483:613–617.
Ursini-Siegel J, Hardy WR, Zuo D, et al. ShcA signalling is essential for tumour progression in mouse models of human breast cancer. EMBO J 2008; 27:910–920.
Debnath J, Muthuswamy SK, Brugge JS . Morphogenesis and oncogenesis of MCF-10A mammary epithelial acini grown in three-dimensional basement membrane cultures. Methods 2003; 30:256–268.
Uhlmann S, Mannsperger H, Zhang JD, et al. Global microRNA level regulation of EGFR-driven cell-cycle protein network in breast cancer. Mol Syst Biol 2012; 8:570.
Acknowledgements
We thank Yu laboratory members for helpful discussions on the manuscript. We thank Novartis for providing BEZ235. We also thank Dr Mark Greene (University of Pennsylvania) for generously providing the hybridoma for 7.16.4 mAb. This study is supported by NIH grants P30-CA 16672 (MDACC), PO1-CA099031 project 4 (DY), RO1-CA112567-07 (DY), Susan G Komen Breast Cancer Foundation Promise Grant KG091020 (DY), Cancer Prevention Research Institute of Texas Grant RP100726 (DY), Career Development Award of MD Anderson Cancer Center Breast SPORE (OS), NIH/NCI pre-doctoral fellowship F31CA165819 (SB) and NIH grant U54CA149169 (STW). D Yu is the Hubert L & Olive Stringer Distinguished Chair in Basic Science at MD Anderson Cancer Center.
Author information
Authors and Affiliations
Corresponding author
Additional information
( Supplementary information is linked to the online version of the paper on the Cell Research website.)
Supplementary information
Supplementary information, Table S1
List of differentially expressed probes between PTEN low/trastuzumab resistant (red) versus PTEN normal/trastuzumab sensitive (blue) breast cancer patients. (XLSX 6366 kb)
Supplementary information, Table S2
List of genes used for IPA to determine canonical pathways differentially enriched between PTEN normal/trastuzumab resistant vs. PTEN high/trastuzumab sensitive breast cancer patients. (PDF 186 kb)
Supplementary information, Table S3
List of proteins with downregulated expression/phosphorylation in Ab7.16.4+lapatinib sensitive PTEN−/−/NIC transplants compared to control and resistant tumors. (PDF 219 kb)
Supplementary information, Table S4
List of antibodies used in western blotting (PDF 182 kb)
Supplementary information, Figure S1
PTEN loss leads to activation of ErbB2 and its downstream signaling in PTEN−/−/NIC mice. (PDF 297 kb)
Supplementary information, Figure S2
TUNEL staining of the tumor tissue obtained from PTEN−/−/NIC mice treated with different drugs or their combination for determination of apoptosis. (PDF 177 kb)
Supplementary information, Figure S3
Syngeneic tumor transplantation model recapitulates the both molecular and biological features of the original PTEN−/−/NIC mice. (PDF 393 kb)
Supplementary information, Figure S4
TUNEL staining of the tumor tissue obtained from PTEN−/−/NIC mice treated with vehicle, Ab+Lapa or sequential A+L/A+B for determination of apoptosis. (PDF 166 kb)
Supplementary information, Figure S5
BEZ235 does not stabilize ErbB2 in 2-D cell culture in BT474.Par or BT474.LapR cells and does not affect ErbB2 mRNA level in 3-D culture. (PDF 265 kb)
Supplementary information, Figure S6
BEZ235 treatment does not significantly alter c-MYC level in 3-D culture. (PDF 153 kb)
Supplementary information, Figure S7
PTEN loss is heterogeneous in Neu-driven, PTEN-loss mouse mammary tumors. (PDF 245 kb)
Rights and permissions
About this article
Cite this article
Sahin, O., Wang, Q., Brady, S. et al. Biomarker-guided sequential targeted therapies to overcome therapy resistance in rapidly evolving highly aggressive mammary tumors. Cell Res 24, 542–559 (2014). https://doi.org/10.1038/cr.2014.37
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/cr.2014.37
Keywords
This article is cited by
-
Exosomal Linc00969 induces trastuzumab resistance in breast cancer by increasing HER-2 protein expression and mRNA stability by binding to HUR
Breast Cancer Research (2023)
-
circCDYL2 promotes trastuzumab resistance via sustaining HER2 downstream signaling in breast cancer
Molecular Cancer (2022)
-
HDAC6 inhibitor TST strengthens the antiproliferative effects of PI3K/mTOR inhibitor BEZ235 in breast cancer cells via suppressing RTK activation
Cell Death & Disease (2018)
-
Translating neoadjuvant therapy into survival benefits: one size does not fit all
Nature Reviews Clinical Oncology (2016)