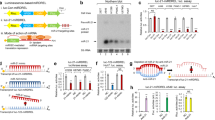
Abstract
Liver and kidney cancers are notorious for drug resistance. Due to the complexity, redundancy and interpatient heterogeneity of resistance mechanisms, most efforts targeting a single pathway were unsuccessful. Novel personalized therapies targeting multiple essential drug resistance pathways in parallel hold a promise for future cancer treatment. Exploiting the multitarget characteristic of microRNAs (miRNAs), we developed a new therapeutic strategy by the combinational use of miRNA and anticancer drugs to increase drug response. By a systems approach, we identified that miR-27b, a miRNA deleted in liver and kidney cancers, sensitizes cancer cells to a broad spectrum of anticancer drugs in vitro and in vivo. Functionally, miR-27b enhances drug response by activating p53-dependent apoptosis and reducing CYP1B1-mediated drug detoxification. Notably, miR-27b promotes drug response specifically in patients carrying p53-wild-type or CYP1B1-high signature. Together, we propose that miR-27b synergizes with anticancer drugs in a defined subgroup of liver and kidney cancer patients.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Holohan C, Van Schaeybroeck S, Longley DB, Johnston PG . Cancer drug resistance: an evolving paradigm. Nat Rev Cancer 2013; 13:714–726.
Corrie PG . Cytotoxic chemotherapy: clinical aspects. Medicine 2008; 36:24–28.
Llovet JM, Ricci S, Mazzaferro V, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med 2008; 359:378–390.
Escudier B, Eisen T, Stadler WM, et al. Sorafenib in advanced clear-cell renal-cell carcinoma. N Engl J Med 2007; 356:125–134.
Chen PJ, Furuse J, Han KH, et al. Issues and controversies of hepatocellular carcinoma-targeted therapy clinical trials in Asia: experts' opinion. Liver Int 2010; 30:1427–1438.
Park JW, Amarapurkar D, Chao Y, et al. Consensus recommendations and review by an International Expert Panel on Interventions in Hepatocellular Carcinoma (EPOIHCC). Liver Int 2013; 33:327–337.
Nanus DM, Garino A, Milowsky MI, Larkin M, Dutcher JP . Active chemotherapy for sarcomatoid and rapidly progressing renal cell carcinoma. Cancer 2004; 101:1545–1551.
Qin S . Guidelines on the diagnosis and treatment of primary liver cancer (2011 edition). Chinese Clinl Oncol 2012; 1.
Tacar O, Sriamornsak P, Dass CR . Doxorubicin: an update on anticancer molecular action, toxicity and novel drug delivery systems. J Pharm Pharmacol 2013; 65:157–170.
Longley DB, Johnston PG . Molecular mechanisms of drug resistance. J Pathol 2005; 205:275–292.
Huang SD, Holzel M, Knijnenburg T, et al. MED12 controls the response to multiple cancer drugs through regulation of TGF-beta receptor signaling. Cell 2012; 151:937–950.
Yap TA, Omlin A, de Bono JS . Development of therapeutic combinations targeting major cancer signaling pathways. J Clin Oncol 2013; 31:1592–1605.
Calin GA, Croce CM . MicroRNA signatures in human cancers. Nat Rev Cancer 2006; 6:857–866.
Bader AG . miR-34 — a microRNA replacement therapy is headed to the clinic. Front Genet 2012; 3:120.
Garzon R, Marcucci G, Croce CM . Targeting microRNAs in cancer: rationale, strategies and challenges. Nat Rev Drug Discov 2010; 9:775–789.
Ling H, Fabbri M, Calin GA . MicroRNAs and other non-coding RNAs as targets for anticancer drug development. Nat Rev Drug Discov 2013; 12:847–865.
Garofalo M, Croce CM . MicroRNAs as therapeutic targets in chemoresistance. Drug Resist Updat 2013; 16:47–59.
Garofalo M, Di Leva G, Romano G, et al. miR-221&222 regulate TRAIL resistance and enhance tumorigenicity through PTEN and TIMP3 downregulation. Cancer Cell 2009; 16:498–509.
Fornari F, Milazzo M, Chieco P, et al. MiR-199a-3p regulates mTOR and c-Met to influence the doxorubicin sensitivity of human hepatocarcinoma cells. Cancer Res 2010; 70:5184–5193.
Ding J, Huang SL, Wang Y, et al. Genome-wide screening reveals that miR-195 targets the TNF-α/NF-κB pathway by down-regulating IκB kinase alpha and TAB3 in hepatocellular carcinoma. Hepatology 2013; 58:654–666.
Knasmuller S, Mersch-Sundermann V, Kevekordes S, et al. Use of human-derived liver cell lines for the detection of environmental and dietary genotoxicants; current state of knowledge. Toxicology 2004; 198:315–328.
Jia D, Dong R, Jing Y, et al. Exome sequencing of hepatoblastoma reveals novel mutations and cancer genes in the Wnt pathway and ubiquitin ligase complex. Hepatology 2014; 60:1686–1696.
Birmingham A, Selfors LM, Forster T, et al. Statistical methods for analysis of high-throughput RNA interference screens. Nat Methods 2009; 6:569–575.
Mitra A, Mishra L, Li SL . Technologies for deriving primary tumor cells for use in personalized cancer therapy. Trends Biotechnol 2013; 31:347–354.
Newell P, Villanueva A, Llovet JM . Molecular targeted therapies in hepatocellular carcinoma: from pre-clinical models to clinical trials. J Hepatol 2008; 49:1–5.
Chin L, Andersen JN, Futreal PA . Cancer genomics: from discovery science to personalized medicine. Nat Med 2011; 17:297–303.
Benson AB 3rd, Abrams TA, Ben-Josef E, et al. NCCN clinical practice guidelines in oncology: hepatobiliary cancers. J Natl Compr Canc Netw 2009; 7:350–391.
Baylin SB, Ohm JE . Epigenetic gene silencing in cancer — a mechanism for early oncogenic pathway addiction? Nat Rev Cancer 2006; 6:107–116.
Szakacs G, Paterson JK, Ludwig JA, Booth-Genthe C, Gottesman MM . Targeting multidrug resistance in cancer. Nat Rev Drug Discov 2006; 5:219–234.
Subramanian A, Tamayo P, Mootha VK, et al. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc Natl Acad Sci USA 2005; 102:15545–15550.
Lewis BP, Shih IH, Jones-Rhoades MW, Bartel DP, Burge CB . Prediction of mammalian microRNA targets. Cell 2003; 115:787–798.
Krek A, Grun D, Poy MN, et al. Combinatorial microRNA target predictions. Nat Genet 2005; 37:495–500.
John B, Enright AJ, Aravin A, Tuschl T, Sander C, Marks DS . human microRNA targets. PLoS Biol 2004; 2:e363.
Okamoto K, Li HY, Jensen MR, et al. Cyclin G recruits PP2A to dephosphorylate Mdm2. Mol Cell 2002; 9:761–771.
Jensen MR, Factor VM, Fantozzi A, Helin K, Huh CG, Thorgeirsson SS . Reduced hepatic tumor incidence in cyclin G1-deficient mice. Hepatology 2003; 37:862–870.
Tsuchiya Y, Nakajima M, Takagi S, Taniya T, Yokoi T . MicroRNA regulates the expression of human cytochrome P4501B1. Cancer Res 2006; 66:9090–9098.
Rochat B, Morsman JM, Murray GI, Figg WD, Mcleod HL . Human CYP1B1 and anticancer agent metabolism: Mechanism for tumor-specific drug inactivation? J Pharmacol Exp Ther 2001; 296:537–541.
Marquardt JU, Thorgeirsson SS . SnapShot: Hepatocellular carcinoma. Cancer Cell 2014; 25:550.e1.
Sotiriou C, Piccart MJ . Opinion — taking gene-expression profiling to the clinic: when will molecular signatures become relevant to patient care? Nat Rev Cancer 2007; 7:545–553.
Miller LD, Smeds J, George J, et al. An expression signature for p53 status in human breast cancer predicts mutation status, transcriptional effects, and patient survival. Proc Natl Acad Sci USA 2005; 102:13550–13555.
Gry M, Rimini R, Stromberg S, et al. Correlations between RNA and protein expression profiles in 23 human cell lines. BMC Genomics 2009; 10:365.
Uhlen M, Oksvold P, Fagerberg L, et al. Towards a knowledge-based human protein atlas. Nat Biotechnol 2010; 28:1248–1250.
Barretina J, Caponigro G, Stransky N, et al. The Cancer cell line encyclopedia enables predictive modelling of anticancer drug sensitivity. Nature 2012; 483:603–607.
Reich M, Liefeld T, Gould J, Lerner J, Tamayo P, Mesirov JP . GenePattern 2.0. Nat Genet 2006; 38:500–501.
Cheng AL, Kang YK, Chen Z, et al. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol 2009; 10:25–34.
Hatziapostolou M, Polytarchou C, Aggelidou E, et al. An HNF4alpha-miRNA inflammatory feedback circuit regulates hepatocellular oncogenesis. Cell 2011; 147:1233–1247.
Hermeking H . MicroRNAs in the p53 network: micromanagement of tumour suppression. Nat Rev Cancer 2012; 12:613–626.
Rukov JL, Shomron N . MicroRNA pharmacogenomics: post-transcriptional regulation of drug response. Trends Mol Med 2011; 17:412–423.
Bartel DP . MicroRNAs: target recognition and regulatory functions. Cell 2009; 136:215–233.
Kasinski AL, Slack FJ . MicroRNAs en route to the clinic: progress in validating and targeting microRNAs for cancer therapy. Nat Rev Cancer 2011; 11:849–864.
Sobrero AF, Maurel J, Fehrenbacher L, et al. EPIC: phase III trial of cetuximab plus irinotecan after fluoropyrimidine and oxaliplatin failure in patients with metastatic colorectal cancer. J Clin Oncol 2008; 26:2311–2319.
Geyer CE, Forster J, Lindquist D, et al. Lapatinib plus capecitabine for HER2-positive advanced breast cancer. N Engl J Med 2006; 355:2733–2743.
Sun TM, Du JZ, Yao YD, et al. Simultaneous delivery of siRNA and paclitaxel via a ”two-in-one” micelleplex promotes synergistic tumor suppression. ACS Nano 2011; 5:1483–1494.
Ohtsuka T, Jensen MR, Kim HG, Kim KT, Lee SW . The negative role of cyclin G in ATM-dependent p53 activation. Oncogene 2004; 23:5405–5408.
Sato Y, Yoshizato T, Shiraishi Y, et al. Integrated molecular analysis of clear-cell renal cell carcinoma. Nat Genet 2013; 45:860–867.
Roe JS, Kim H, Lee SM, Kim ST, Cho EJ, Youn HD . p53 stabilization and transactivation by a von Hippel-Lindau protein. Mol Cell 2006; 22:395–405.
McFadyen MC, Melvin WT, Murray GI . Cytochrome P450 enzymes: novel options for cancer therapeutics. Mol Cancer Ther 2004; 3:363–371.
McFadyen MC, Murray GI . Cytochrome P450 1B1: a novel anticancer therapeutic target. Future Oncol 2005; 1:259–263.
Offer SM, Butterfield GL, Jerde CR, Fossum CC, Wegner NJ, Diasio RB . microRNAs miR-27a and miR-27b directly regulate liver dihydropyrimidine dehydrogenase expression through two conserved binding sites. Mol Cancer Ther 2014; 13:742–751.
Al-Lazikani B, Banerji U, Workman P . Combinatorial drug therapy for cancer in the post-genomic era. Nat Biotechnol 2012; 30:679–691.
Sharma SV, Haber DA, Settleman J . Cell line-based platforms to evaluate the therapeutic efficacy of candidate anticancer agents. Nat Rev Cancer 2010; 10:241–253.
Acknowledgements
We are grateful to Drs Bakiri Latifa, Mofang Liu, Ruimin Huang, Erwei Song, Hai Jiang, Hongbin Ji, and Lihua Min for critical comments on the manuscript. This study was funded by the National Natural Science Foundation of China (31225016, 81170418 and 81125016), the Ministry of Science and Technology of China (2014CB910601, 2011ZX09307-302-01, 2011CB966304 and 2011CB910204), the Science and Technology Commission of Shanghai Municipality (12JC1409500 and 14XD1404200), and the Natural Science Foundation of Jiangsu Province (BK20131084).
Author information
Authors and Affiliations
Corresponding authors
Additional information
( Supplementary information is linked to the online version of the paper on the Cell Research website.)
Supplementary information
Supplementary information, Figure S1
Identification of miRNAs that enhance drug sensitivity by functional screen. (PDF 157 kb)
Supplementary information, Figure S2
miR-27b is the master miRNA for enhancing drug sensitivity in liver cancers. (PDF 1032 kb)
Supplementary information, Figure S3
Expression levels of drug sensitizing miRNAs and miR-23b∼27b∼24-1 gene cluster members in liver cancers. (PDF 1583 kb)
Supplementary information, Figure S4
The combinational therapy of miR-27b and doxorubicin shows synergistic therapeutic effect on liver cancers and kidney cancers in vivo. (PDF 3618 kb)
Supplementary information, Figure S5
miR-27b enhances the drug response by activating p53. (PDF 263 kb)
Supplementary information, Figure S6
miR-27b directly targets CCNG1 to activate p53 activity. (PDF 109 kb)
Supplementary information, Figure S7
The miR-27b-CCNG1-p53 mechanism accounted for the regulation of sensitivity to sorafenib. (PDF 225 kb)
Supplementary information, Figure S8
Knockdown of CCNG1 did not confer drug sensitivity in p53 mutant cell lines. (PDF 92 kb)
Supplementary information, Figure S9
CYP1B1 is post-transcriptionally regulated by miR-27b. (PDF 762 kb)
Supplementary information, Figure S10
miR-27b promotes drug sensitivity by additionally reducing CYP1B1 levels. (PDF 134 kb)
Supplementary information, Figure S11
The establishment of the CYP1B1 gene signature. (PDF 215 kb)
Supplementary information, Figure S12
miR-27b promotes good drug responses in patients with wild-type p53 or high CYP1B1 levels. (PDF 147 kb)
Supplementary information, Table S1
miRNAs promoting HepG2 cells sensitive or resistant to doxorubicin in the high-throughput screen (PDF 28 kb)
Supplementary information, Table S2
miRNAs increasing or decreasing HepG2 cell numbers in the high-throughput screen (PDF 28 kb)
Supplementary information, Table S3
Pathways regulated by each miRNA in the miRNA-pathway network (PDF 37 kb)
Supplementary information, Table S4
Information of 49 paired liver cancers and 66 paired kidney cancers (PDF 64 kb)
Supplementary information, Table S5
The original data of subcutaneously transplanted tumors (PDF 14 kb)
Supplementary information, Table S6
Fold change of genes upon forced miR-27b expression (PDF 7 kb)
Supplementary information, Table S7
Fold change of genes upon forced miR-27b expression (PDF 9 kb)
Supplementary information, Table S8
p53 status of different cancer cell lines (PDF 6 kb)
Supplementary information, Table S9
CYP1B1 signature genes (PDF 7 kb)
Supplementary information, Table S10
Information of 29 liver cancer patients and 60 kidney cancer patients with drug treatment (PDF 12 kb)
Supplementary information, Table S11
Primers for vector construction (PDF 6 kb)
Supplementary information, Data S1
Supplemenal Methods (PDF 152 kb)
Rights and permissions
About this article
Cite this article
Mu, W., Hu, C., Zhang, H. et al. miR-27b synergizes with anticancer drugs via p53 activation and CYP1B1 suppression. Cell Res 25, 477–495 (2015). https://doi.org/10.1038/cr.2015.23
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/cr.2015.23
Keywords
This article is cited by
-
Hsa-microRNA-27b-3p inhibits hepatocellular carcinoma progression by inactivating transforming growth factor-activated kinase-binding protein 3/nuclear factor kappa B signalling
Cellular & Molecular Biology Letters (2022)
-
MicroRNAs’ role in the environment-related non-communicable diseases and link to multidrug resistance, regulation, or alteration
Environmental Science and Pollution Research (2021)
-
Biological roles of cytochrome P450 1A1, 1A2, and 1B1 enzymes
Archives of Pharmacal Research (2021)
-
Tumor-suppressor miRNA-27b-5p regulates the growth and metastatic behaviors of ovarian carcinoma cells by targeting CXCL1
Journal of Ovarian Research (2020)
-
The emerging role of microRNAs and long noncoding RNAs in drug resistance of hepatocellular carcinoma
Molecular Cancer (2019)