Abstract
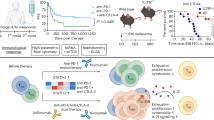
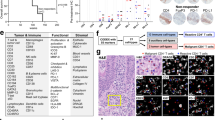
The cytotoxic T lymphocyte antigen-4 (CTLA-4)-blocking antibody ipilimumab induces immune-mediated long-term control of metastatic melanoma in a fraction of patients. Although ipilimumab undoubtedly exerts its therapeutic effects via immunostimulation, thus far clinically useful, immunologically relevant biomarkers that predict treatment efficiency have been elusive. Here, we show that neutralization of IL-2 or blocking the α and β subunits of the IL-2 receptor (CD25 and CD122, respectively) abolished the antitumor effects and the accompanying improvement of the ratio of intratumoral T effector versus regulatory cells (Tregs), which were otherwise induced by CTLA-4 blockade in preclinical mouse models. CTLA-4 blockade led to the reduction of a suppressive CD4+ T cell subset expressing Lag3, ICOS, IL-10 and Egr2 with a concomitant rise in IL-2-producing effector cells that lost FoxP3 expression and accumulated in regressing tumors. While recombinant IL-2 improved the therapeutic efficacy of CTLA-4 blockade, the decoy IL-2 receptor α (IL-2Rα, sCD25) inhibited the anticancer effects of CTLA-4 blockade. In 262 metastatic melanoma patients receiving ipilimumab, baseline serum concentrations of sCD25 represented an independent indicator of overall survival, with high levels predicting resistance to therapy. Altogether, these results unravel a role for IL-2 and IL-2 receptors in the anticancer activity of CTLA-4 blockade. Importantly, our study provides the first immunologically relevant biomarker, namely elevated serum sCD25, that predicts resistance to CTLA-4 blockade in patients with melanoma.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
Abbreviations
- CTLA-4:
-
cytotoxic T lymphocyte antigen-4
- MM:
-
metastatic melanoma
- MoA:
-
mode of action
- Lag3:
-
lymphocyte-activation gene 3
- ICOS:
-
inducible T-cell costimulator
- ICOSL:
-
ICOS ligand
- Egr-2:
-
early growth response gene-2
- rIL-2:
-
recombinant interleukin 2
- sCD25:
-
soluble CD25 (IL-2 receptor alpha chain)
- Th:
-
T helper
- GM-CSF:
-
granulocyte-macrophage colony-stimulating factor
- GVAX:
-
GM-CSF gene-transfected tumor cell vaccine
- Treg:
-
regulatory T cell
- Teff:
-
effector T cell
- MICA:
-
MHC class I-related chain A
- IFNAR:
-
interferon-α/β receptor
- R:
-
responder
- NR:
-
non responder
- OS:
-
overall survival
- ir-RC:
-
immune response-related criteria
- TCR:
-
T-cell receptor
- TILs:
-
tumor-infiltrating lymphocytes
References
Schreiber RD, Old LJ, Smyth MJ . Cancer immunoediting: integrating immunity's roles in cancer suppression and promotion. Science 2011; 331:1565–1570.
Fridman WH, Pages F, Sautes-Fridman C, Galon J . The immune contexture in human tumours: impact on clinical outcome. Nat Rev Cancer 2012; 12:298–306.
Bour-Jordan H, Esensten JH, Martinez-Llordella M, et al. Intrinsic and extrinsic control of peripheral T-cell tolerance by costimulatory molecules of the CD28/ B7 family. Immunol Rev 2011; 241:180–205.
Hodi FS, O'Day SJ, McDermott DF, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med 2010; 363:711–723.
Robert C, Thomas L, Bondarenko I, et al. Ipilimumab plus dacarbazine for previously untreated metastatic melanoma. N Engl J Med 2011; 364:2517–2526.
Krummel MF, Allison JP . CD28 and CTLA-4 have opposing effects on the response of T cells to stimulation. J Exp Med 1995; 182:459–465.
Peggs KS, Quezada SA, Allison JP . Cell intrinsic mechanisms of T-cell inhibition and application to cancer therapy. Immunol Rev 2008; 224:141–165.
Blair PJ, Riley JL, Levine BL, et al. CTLA-4 ligation delivers a unique signal to resting human CD4 T cells that inhibits interleukin-2 secretion but allows Bcl-X(L) induction. J Immunol 1998; 160:12–15.
Chen W, Jin W, Wahl SM . Engagement of cytotoxic T lymphocyte-associated antigen 4 (CTLA-4) induces transforming growth factor beta (TGF-beta) production by murine CD4(+) T cells. J Exp Med 1998; 188:1849–1857.
Read S, Malmstrom V, Powrie F . Cytotoxic T lymphocyte-associated antigen 4 plays an essential role in the function of CD25(+)CD4(+) regulatory cells that control intestinal inflammation. J Exp Med 2000; 192:295–302.
Wing K, Onishi Y, Prieto-Martin P, et al. CTLA-4 control over Foxp3+ regulatory T cell function. Science 2008; 322:271–275.
Simpson TR, Li F, Montalvo-Ortiz W, et al. Fc-dependent depletion of tumor-infiltrating regulatory T cells co-defines the efficacy of anti-CTLA-4 therapy against melanoma. J Exp Med 2013; 210:1695–1710.
Vanneman M, Dranoff G . Combining immunotherapy and targeted therapies in cancer treatment. Nat Rev Cancer 2012; 12:237–251.
Wada S, Jackson CM, Yoshimura K, et al. Sequencing CTLA-4 blockade with cell-based immunotherapy for prostate cancer. J Transl Med 2013; 11:89.
Holmgaard RB, Zamarin D, Munn DH, Wolchok JD, Allison JP . Indoleamine 2,3-dioxygenase is a critical resistance mechanism in antitumor T cell immunotherapy targeting CTLA-4. J Exp Med 2013; 210:1389–1402.
Lesterhuis WJ, Salmons J, Nowak AK, et al. Synergistic effect of CTLA-4 blockade and cancer chemotherapy in the induction of anti-tumor immunity. PLoS One 2013; 8:e61895.
Delyon J, Mateus C, Lefeuvre D, et al. Experience in daily practice with ipilimumab for the treatment of patients with metastatic melanoma: an early increase in lymphocyte and eosinophil counts is associated with improved survival. Ann Oncol 2013; 24:1697–1703.
Liakou CI, Kamat A, Tang DN, et al. CTLA-4 blockade increases IFNgamma-producing CD4+ICOShi cells to shift the ratio of effector to regulatory T cells in cancer patients. Proc Natl Acad Sci USA 2008; 105:14987–14992.
Maker AV, Attia P, Rosenberg SA . Analysis of the cellular mechanism of antitumor responses and autoimmunity in patients treated with CTLA-4 blockade. J Immunol 2005; 175:7746–7754.
Yuan J, Adamow M, Ginsberg BA, et al. Integrated NY-ESO-1 antibody and CD8+ T-cell responses correlate with clinical benefit in advanced melanoma patients treated with ipilimumab. Proc Natl Acad Sci USA 2011; 108:16723–16728.
Hodi FS, Butler M, Oble DA, et al. Immunologic and clinical effects of antibody blockade of cytotoxic T lymphocyte-associated antigen 4 in previously vaccinated cancer patients. Proc Natl Acad Sci USA 2008; 105:3005–3010.
Hamid O, Schmidt H, Nissan A, et al. A prospective phase II trial exploring the association between tumor microenvironment biomarkers and clinical activity of ipilimumab in advanced melanoma. J Transl Med 2011; 9:204.
Ribas A, Comin-Anduix B, Economou JS, et al. Intratumoral immune cell infiltrates, FoxP3, and indoleamine 2,3-dioxygenase in patients with melanoma undergoing CTLA4 blockade. Clin Cancer Res 2009; 15:390–399.
Chen H, Liakou CI, Kamat A, et al. Anti-CTLA-4 therapy results in higher CD4+ICOShi T cell frequency and IFN-gamma levels in both nonmalignant and malignant prostate tissues. Proc Natl Acad Sci USA 2009; 106:2729–2734.
Jinushi M, Hodi FS, Dranoff G . Therapy-induced antibodies to MHC class I chain-related protein A antagonize immune suppression and stimulate antitumor cytotoxicity. Proc Natl Acad Sci USA 2006; 103:9190–9195.
Ji RR, Chasalow SD, Wang L, et al. An immune-active tumor microenvironment favors clinical response to ipilimumab. Cancer Immunol Immunother 2012; 61:1019–1031.
Yuan J, Zhou J, Dong Z, et al. Pretreatment serum VEGF is associated with clinical response and overall survival in advanced melanoma patients treated with Ipilimumab. Cancer Immunol Res 2014; 2:127–132.
Shrikant P, Khoruts A, Mescher MF . CTLA-4 blockade reverses CD8+ T cell tolerance to tumor by a CD4+ T cell- and IL-2-dependent mechanism. Immunity 1999; 11:483–493.
Maker AV, Phan GQ, Attia P, et al. Tumor regression and autoimmunity in patients treated with cytotoxic T lymphocyte-associated antigen 4 blockade and interleukin 2: a phase I/II study. Ann Surg Oncol 2005; 12:1005–1016.
Cabrera R, Ararat M, Eksioglu EA, et al. Influence of serum and soluble CD25 (sCD25) on regulatory and effector T-cell function in hepatocellular carcinoma. Scand J Immunol 2010; 72:293–301.
Gooding R, Riches P, Dadian G, Moore J, Gore M . Increased soluble interleukin-2 receptor concentration in plasma predicts a decreased cellular response to IL-2. Br J Cancer 1995; 72:452–455.
Eggermont AM, Suciu S, Testori A, et al. Long-term results of the randomized phase III trial EORTC 18991 of adjuvant therapy with pegylated interferon alfa-2b versus observation in resected stage III melanoma. J Clin Oncol 2012; 30:3810–3818.
Tourani JM, Pfister C, Tubiana N, et al. Subcutaneous interleukin-2 and interferon alfa administration in patients with metastatic renal cell carcinoma: final results of SCAPP III, a large, multicenter, phase II, nonrandomized study with sequential analysis design — the Subcutaneous Administration Propeukin Program Cooperative Group. J Clin Oncol 2003; 21:3987–3994.
Negrier S, Perol D, Ravaud A, et al. Randomized study of intravenous versus subcutaneous interleukin-2, and IFNalpha in patients with good prognosis metastatic renal cancer. Clin Cancer Res 2008; 14:5907–5912.
Lotze MT, Matory YL, Ettinghausen SE, et al. In vivo administration of purified human interleukin 2. II. Half life, immunologic effects, and expansion of peripheral lymphoid cells in vivo with recombinant IL 2. J Immunol 1985; 135:2865–2875.
Okamura T, Fujio K, Sumitomo S, Yamamoto K . Roles of LAG3 and EGR2 in regulatory T cells. Ann Rheum Dis 2012; 71 Suppl 2:i96–i100.
Saadoun D, Rosenzwajg M, Joly F, et al. Regulatory T-cell responses to low-dose interleukin-2 in HCV-induced vasculitis. N Engl J Med 2011; 365:2067–2077.
Bien E, Balcerska A . Serum soluble interleukin 2 receptor alpha in human cancer of adults and children: a review. Biomarkers 2008; 13:1–26.
Kavanagh B, O'Brien S, Lee D, et al. CTLA4 blockade expands FoxP3+ regulatory and activated effector CD4+ T cells in a dose-dependent fashion. Blood 2008; 112:1175–1183.
Balch CM, Gershenwald JE, Soong SJ, et al. Final version of 2009 AJCC melanoma staging and classification. J Clin Oncol 2009; 27:6199–6206.
Mellman I, Coukos G, Dranoff G . Cancer immunotherapy comes of age. Nature 2011; 480:480–489.
Waterhouse P, Penninger JM, Timms E, et al. Lymphoproliferative disorders with early lethality in mice deficient in Ctla-4. Science 1995; 270:985–988.
Quezada SA, Peggs KS, Curran MA, Allison JP . CTLA4 blockade and GM-CSF combination immunotherapy alters the intratumor balance of effector and regulatory T cells. J Clin Invest 2006; 116:1935–1945.
Hannier S, Tournier M, Bismuth G, Triebel F . CD3/TCR complex-associated lymphocyte activation gene-3 molecules inhibit CD3/TCR signaling. J Immunol 1998; 161:4058–4065.
Gagliani N, Magnani CF, Huber S, et al. Coexpression of CD49b and LAG-3 identifies human and mouse T regulatory type 1 cells. Nat Med 2013; 19:739–746.
Zheng Y, Zha Y, Driessens G, Locke F, Gajewski TF . Transcriptional regulator early growth response gene 2 (Egr2) is required for T cell anergy in vitro and in vivo. J Exp Med 2012; 209:2157–2163.
Lotze MT, Custer MC, Sharrow SO, et al. In vivo administration of purified human interleukin-2 to patients with cancer: development of interleukin-2 receptor positive cells and circulating soluble interleukin-2 receptors following interleukin-2 administration. Cancer Res 1987; 47:2188–2195.
Boyano MD, Garcia-Vazquez MD, Lopez-Michelena T, et al. Soluble interleukin-2 receptor, intercellular adhesion molecule-1 and interleukin-10 serum levels in patients with melanoma. Br J Cancer 2000; 83:847–852.
Lissoni P, Tancini G, Rovelli F, et al. Serum interleukin-2 levels in relation to the neuroendocrine status in cancer patients. Br J Cancer 1990; 62:838–839.
Nakase K, Tsuji K, Tamaki S, et al. Elevated levels of soluble interleukin-2 receptor in serum of patients with hematological or non-hematological malignancies. Cancer Detect Prev 2005; 29:256–259.
Ottaiano A, Leonardi E, Simeone E, et al. Soluble interleukin-2 receptor in stage I-III melanoma. Cytokine 2006; 33:150–155.
Sabbioni ME, Siegrist HP, Bacchi M, et al. Association between immunity and prognostic factors in early stage breast cancer patients before adjuvant treatment. Breast Cancer Res Treat 2000; 59:279–287.
Maier LM, Anderson DE, Severson CA, et al. Soluble IL-2RA levels in multiple sclerosis subjects and the effect of soluble IL-2RA on immune responses. J Immunol 2009; 182:1541–1547.
Symons JA, Wood NC, Di Giovine FS, Duff GW . Soluble IL-2 receptor in rheumatoid arthritis. Correlation with disease activity, IL-1 and IL-2 inhibition. J Immunol 1988; 141:2612–2618.
Zorn U, Dallmann I, Grosse J, et al. Soluble interleukin 2 receptors abrogate IL-2 induced activation of peripheral mononuclear cells. Cytokine 1994; 6:358–364.
Russell SE, Moore AC, Fallon PG, Walsh PT . Soluble IL-2Ralpha (sCD25) exacerbates autoimmunity and enhances the development of Th17 responses in mice. PLoS One 2012; 7:e47748.
Wilgenhof S, Du Four S, Vandenbroucke F, et al. Single-center experience with ipilimumab in an expanded access program for patients with pretreated advanced melanoma. J Immunother 2013; 36:215–222.
Kelderman S, Heemskerk B, van Tinteren H, et al. Lactate dehydrogenase as a selection criterion for ipilimumab treatment in metastatic melanoma. Cancer Immunol Immunother 2014; 63:449–458.
Morris JC, Waldmann TA . Advances in interleukin 2 receptor targeted treatment. Ann Rheum Dis 2000; 59 Suppl 1:i109–i114.
Peggs KS, Quezada SA, Chambers CA, Korman AJ, Allison JP . Blockade of CTLA-4 on both effector and regulatory T cell compartments contributes to the antitumor activity of anti-CTLA-4 antibodies. J Exp Med 2009; 206:1717–1725.
Sugar E, Pascoe AJ, Azad N . Reporting of preclinical tumor-graft cancer therapeutic studies. Cancer Biol Ther 2012; 13:1262–1268.
Acknowledgements
We thank Philippe Rameau and Yann Lécluse for assistance in FACS and cell sorting experiments and Michelle Yong for help with mechanism studies in the mouse models. We are grateful to the staff of the animal facility of Gustave Roussy. DH was supported by ARC while l'Oreal awarded a prize to MV. GK and LZ were supported by the Ligue Nationale contre le Cancer (Equipes labellisées), SIRIC Socrates, Agence Nationale pour la Recherche (ANR AUTOPH, ANR Emergence), European Commission (ArtForce), European Research Council Advanced Investigator Grant (to GK), Fondation pour la Recherche Médicale (FRM), Institut National du Cancer (INCa), Fondation de France, Cancéropôle Ile-de-France, Fondation Bettencourt-Schueller, the LabEx Immuno-Oncology, the SIRIC Stratified Oncology Cell DNA Repair and Tumor Immune Elimination (SOCRATE), the SIRIC Cancer Research and Personalized Medicine (CARPEM), and the Paris Alliance of Cancer Research Institutes (PACRI). MJS was supported by a National Health and Medical Research Council (NH&MRC) Australia Fellowship and Program Grant and a program grant from the Victorian Cancer Agency. MWLT was supported by a NH&MRC Career Development Fellowship. MM was supported by the Associazione Italiana per la Ricerca sul Cancro.
Author information
Authors and Affiliations
Corresponding authors
Additional information
( Supplementary information is linked to the online version of the paper on the Cell Research website.)
Supplementary information
Supplementary information, Figure S1
Contributions of IFNAR, CD4+T, CD8+T, NK cells and NKG2D to the antitumor effects of CTLA-4 blockade. (PDF 324 kb)
Supplementary information, Figure S2
Monitoring of Foxp3 CD4+ T cells during mCTLA4 blockade. (PDF 175 kb)
Supplementary information, Figure S3
Effects of mCTLA-4 blockade or sCD25 injections on serum sCD25 concentrations. (PDF 52 kb)
Supplementary information, Figure S4
Univariate analyses of multiple parameters of clinical significance for OS of MM patients. (PDF 255 kb)
Supplementary information, Figure S5
sCD25 serum levels at T0 versus T21 and OS in MM patients. (PDF 256 kb)
Supplementary information, Table S1
Patients characteristics (DOC 69 kb)
Rights and permissions
About this article
Cite this article
Hannani, D., Vétizou, M., Enot, D. et al. Anticancer immunotherapy by CTLA-4 blockade: obligatory contribution of IL-2 receptors and negative prognostic impact of soluble CD25. Cell Res 25, 208–224 (2015). https://doi.org/10.1038/cr.2015.3
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/cr.2015.3
Keywords
This article is cited by
-
Cancer immunotherapy: it’s time to better predict patients’ response
British Journal of Cancer (2021)
-
Biomarkers Associated with Clinical Outcome of Advanced Melanoma Patients Treated with Ipilimumab
Pathology & Oncology Research (2020)
-
Lymphopenia in Cancer Patients and its Effects on Response to Immunotherapy: an opportunity for combination with Cytokines?
Journal for ImmunoTherapy of Cancer (2019)
-
Monitoring checkpoint inhibitors: predictive biomarkers in immunotherapy
Frontiers of Medicine (2019)
-
Existing and Emerging Biomarkers for Immune Checkpoint Immunotherapy in Solid Tumors
Advances in Therapy (2019)