Abstract
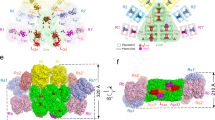
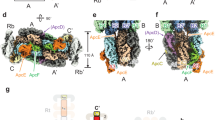
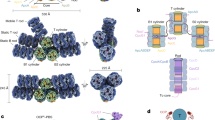
Phycobilisomes (PBSs) are light-harvesting antennae that transfer energy to photosynthetic reaction centers in cyanobacteria and red algae. PBSs are supermolecular complexes composed of phycobiliproteins (PBPs) that bear chromophores for energy absorption and linker proteins. Although the structures of some individual components have been determined using crystallography, the three-dimensional structure of an entire PBS complex, which is critical for understanding the energy transfer mechanism, remains unknown. Here, we report the structures of an intact PBS and a PBS in complex with photosystem II (PSII) from Anabaena sp. strain PCC 7120 using single-particle electron microscopy in combination with biochemical and molecular analyses. In the PBS structure, all PBP trimers and the conserved linker protein domains were unambiguously located, and the global distribution of all chromophores was determined. We provide evidence that ApcE and ApcF are critical for the formation of a protrusion at the bottom of PBS, which plays an important role in mediating PBS interaction with PSII. Our results provide insights into the molecular architecture of an intact PBS at different assembly levels and provide the basis for understanding how the light energy absorbed by PBS is transferred to PSII.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
MacColl R . Cyanobacterial phycobilisomes. J Struct Biol 1998; 124:311–334.
Sidler WA . Phycobilisome and phycobiliprotein structures. In: Bryant DA, ed. The Molecular Biology of Cyanobacteria. The Netherlands: Kluwer Academic Publishers, 1994:139–216.
Adir N . Elucidation of the molecular structures of components of the phycobilisome: reconstructing a giant. Photosynth Res 2005; 85:15–32.
Glauser M, Bryant DA, Frank G, et al. Phycobilisome structure in the cyanobacteria Mastigocladus laminosus and Anabaena sp. PCC 7120. Eur J Biochem 1992; 205:907–915.
Ducret A, Sidler W, Wehrli E, Frank G, Zuber H . Isolation, characterization and electron microscopy analysis of a hemidiscoidal phycobilisome type from the cyanobacterium Anabaena sp. PCC 7120. Eur J Biochem 1996; 236:1010–1024.
Ducret A, Muller SA, Goldie KN, et al. Reconstitution, characterisation and mass analysis of the pentacylindrical allophycocyanin core complex from the cyanobacterium Anabaena sp. PCC 7120. J Mol Biol 1998; 278:369–388.
Anderson LK, Toole CM . A model for early events in the assembly pathway of cyanobacterial phycobilisomes. Mol Microbiol 1998; 30:467–474.
Schirmer T, Bode W, Huber R . Refined three-dimensional structures of two cyanobacterial C-phycocyanins at 2.1 and 2.5 Å resolution. A common principle of phycobilin-protein interaction. J Mol Biol 1987; 196:677–695.
Schirmer T, Huber R, Schneider M, Bode W, Miller M, Hackert ML . Crystal structure analysis and refinement at 2.5 Å of hexameric C-phycocyanin from the cyanobacterium Agmenellum quadruplicatum. The molecular model and its implications for light-harvesting. J Mol B 1986; 188:651–676.
Reuter W, Wiegand G, Huber R, Than ME . Structural analysis at 2.2 Å of orthorhombic crystals presents the asymmetry of the allophycocyanin-linker complex, AP.LC7.8, from phycobilisomes of Mastigocladus laminosus. Proc Natl Acad Sci USA 1999; 96:1363–1368.
Guan X, Qin S, Zhao F, Zhang X, Tang X . Phycobilisomes linker family in cyanobacterial genomes: divergence and evolution. Int J Biol Sci 2007; 3:434–445.
Wilk KE, Harrop SJ, Jankova L, et al. Evolution of a light-harvesting protein by addition of new subunits and rearrangement of conserved elements: crystal structure of a cryptophyte phycoerythrin at 1.63-Å resolution. Proc Natl Acad Sci USA 1999; 96:8901–8906.
Ritter S, Hiller RG, Wrench PM, Welte W, Diederichs K . Crystal structure of a phycourobilin-containing phycoerythrin at 1.90-Å resolution. J Struct Biol 1999; 126:86–97.
Nield J, Rizkallah PJ, Barber J, Chayen NE . The 1.45 Å three-dimensional structure of C-phycocyanin from the thermophilic cyanobacterium Synechococcus elongatus. J Struct Biol 2003; 141:149–155.
David L, Marx A, Adir N . High-resolution crystal structures of trimeric and rod phycocyanin. J Mol Biol 2011; 405:201–213.
Stec B, Troxler RF, Teeter MM . Crystal structure of C-phycocyanin from Cyanidium caldarium provides a new perspective on phycobilisome assembly. Biophys J 1999; 76:2912–2921.
Marx A, Adir N . Allophycocyanin and phycocyanin crystal structures reveal facets of phycobilisome assembly. Biochim Biophys Acta 2013; 1827:311–318.
Liu JY, Jiang T, Zhang JP, Liang DC . Crystal structure of allophycocyanin from red algae Porphyra yezoensis at 2.2-Å resolution. J Biol Chem 1999; 274:16945–16952.
Murray JW, Maghlaoui K, Barber J . The structure of allophycocyanin from Thermosynechococcus elongatus at 3.5 Å resolution. Acta Crystallogr Sect F Struct Biol Cryst Commun 2007; 63:998–1002.
Gao X, Zhang N, Wei TD, et al. Crystal structure of the N-terminal domain of linker L(R) and the assembly of cyanobacterial phycobilisome rods. Mol Microbiol 2011; 82:698–705.
Arteni AA, Ajlani G, Boekema EJ . Structural organization of phycobilisomes from Synechocystis sp. strain PCC6803 and their interaction with the membrane. Biochim Biophys Acta 2009; 1787:272–279.
Yi ZW, Huang H, Kuang TY, Sui SF . Three-dimensional architecture of phycobilisomes from Nostoc flagelliforme revealed by single particle electron microscopy. FEBS Lett 2005; 579:3569–3573.
Murata N . Control of excitation transfer in photosynthesis. I. Light-induced change of chlorophyll a fluorescence in Porphyridium cruentum. Biochim Biophys Acta 1969; 172:242–251.
Fork DC, Satoh K . State I-state II transitions in the thermophilic blue-green alga (cyanobacterium) Synechococcus lividus. Photochem Photobiol 1983; 37:421–427.
Mohanty P, Govindjee . Light-induced changes in the fluorescence yield of chlorophyll a in Anacystis nidulans II. The fast changes and the effect of photosynthetic inhibitors on both the fast and slow fluorescence induction. Plant Cell Physiol 1973; 14:611–629.
Mullineaux CW, Allen JF . State 1-State 2 transitions in the cyanobacterium Synechococcus 6301 are controlled by the redox state of electron carriers between Photosystems I and II. Photosynth Res 1990; 23:297–311.
Liu H, Zhang H, Niedzwiedzki DM, et al. Phycobilisomes supply excitations to both photosystems in a megacomplex in cyanobacteria. Science 2013; 342:1104–1107.
Giddings TH, Wasmann C, Staehelin LA . Structure of the thylakoids and envelope membranes of the cyanelles of Cyanophora paradoxa. Plant Physiol 1983; 71:409–419.
Mullineaux CW . Phycobilisome-reaction center interaction in cyanobacteria. Photosynth Res 2008; 95:175–182.
Barber J, Morris EP, da Fonseca PC . Interaction of the allophycocyanin core complex with photosystem II. Photochem Photobiol Sci 2003; 2:536–541.
Chereskin BM, Clement-Metral JD, Gantt E . Characterization of a purified photosystem II-phycobilisome particle preparation from Porphyridium cruentum. Plant Physiol 1985; 77:626–629.
Gantt E, Clement-Metral JD, Chereskin BM . Methods in Enzymology. San Diego: Academic Press, 1988.
Guskov A, Kern J, Gabdulkhakov A, Broser M, Zouni A, Saenger W . Cyanobacterial photosystem II at 2.9-Å resolution and the role of quinones, lipids, channels and chloride. Nat Struct Mol Biol 2009; 16:334–342.
Umena Y, Kawakami K, Shen JR, Kamiya N . Crystal structure of oxygen-evolving photosystem II at a resolution of 1.9 Å. Nature 2011; 473:55–60.
Ajlani G, Vernotte C . Deletion of the PB-loop in the L(CM) subunit does not affect phycobilisome assembly or energy transfer functions in the cyanobacterium Synechocystis sp. PCC6714. Eur J Biochem 1998; 257:154–159.
Capuano V, Braux AS, Tandeau de Marsac N, Houmard J . The “anchor polypeptide” of cyanobacterial phycobilisomes. Molecular characterization of the Synechococcus sp. PCC 6301 apce gene. J Biol Chem 1991; 266:7239–7247.
Satyanarayana L, Suresh CG, Patel A, Mishra S, Ghosh PK . X-ray crystallographic studies on C-phycocyanins from cyanobacteria from different habitats: marine and freshwater. Acta Crystallogr Sec F Struct Biol Cryst Commun 2005; 61:844–847.
David L, Prado M, Arteni AA, Elmlund DA, Blankenship RE, Adir N . Structural studies show energy transfer within stabilized phycobilisomes independent of the mode of rod-core assembly. Biochim Biophys Acta 2014; 1837:385–395.
Watanabe M, Semchonok DA, Webber-Birungi MT, et al. Attachment of phycobilisomes in an antenna-photosystem I supercomplex of cyanobacteria. Proc Natl Acad Sci USA 2014; 111:2512–2517.
Rigbi M, Rosinski J, Siegelman HW, Sutherland JC . Cyanobacterial phycobilisomes: Selective dissociation monitored by fluorescence and circular dichroism. Proc Natl Acad Sci USA 1980; 77:1961–1965.
Gindt YM, Zhou J, Bryant DA, Sauer K . Core mutations of Synechococcus sp. PCC 7002 phycobilisomes: a spectroscopic study. J Photochem Photobiol B 1992; 15:75–89.
Bryant DA, Stirewalt VL, Glauser M, Frank G, Sidler W, Zuber H . A small multigene family encodes the rod-core linker polypeptides of Anabaena sp. PCC7120 phycobilisomes. Gene 1991; 107:91–99.
Dong C, Tang A, Zhao J, Mullineaux CW, Shen G, Bryant DA . ApcD is necessary for efficient energy transfer from phycobilisomes to photosystem I and helps to prevent photoinhibition in the cyanobacterium Synechococcus sp. PCC 7002. Biochim Biophys Acta 2009; 1787:1122–1128.
Bryant DA . Genetic analysis of phycobilisome biosynthesis, assembly, structure, and function in the Cyanobacterium Synechococcus sp. PCC 7002. In: Stevens SE, Bryant DA. ed. Light-Energy Transduction in Photosynthesis: HigherPlant and Bacterial Models. Rockville, Maryland, USA: American Society of Plant Physiologists 1988:62–90.
Ludtke SJ, Baldwin PR, Chiu W . EMAN: semiautomated software for high-resolution single-particle reconstructions. J Struct Biol 1999; 128:82–97.
Frank J, Radermacher M, Penczek P, et al. SPIDER and WEB: processing and visualization of images in 3D electron microscopy and related fields. J Struct Biol 1996; 116:190–199.
Pettersen EF, Goddard TD, Huang CC, et al. UCSF Chimera — a visualization system for exploratory research and analysis. J Comput Chem 2004; 25:1605–1612.
Tichy M, Lupinkova L, Sicora C, et al. Synechocystis 6803 mutants expressing distinct forms of the Photosystem II D1 protein from Synechococcus 7942: relationship between the psbA coding region and sensitivity to visible and UV-B radiation. Biochim Biophys Acta 2003; 1605:55–66.
Bajkan S, Varadi G, Balogh M, et al. Conserved structure of the chloroplast-DNA encoded D1 protein is essential for effective photoprotection via non-photochemical thermal dissipation in higher plants. Mol Genet Genomics 2010; 284:55–63.
Toth T, Zsiros O, Kis M, Garab G, Kovacs L . Cadmium exerts its toxic effects on photosynthesis via a cascade mechanism in the cyanobacterium, Synechocystis PCC 6803. Plant Cell Environ 2012; 35:2075–2086.
Acknowledgements
We thank Jianlin Lei for setting up a semi-automated program for data collection, Lixin Zhang for providing antibodies against D1 and D2, Ying-Chun Hu for technical assistance in immunogold labeling experiments, and Hong-Wei Wang and Da-Neng Wang for critical reading of the manuscript. We acknowledge the Tsinghua University Branch of China National Center for Protein Sciences Beijing for providing EM resources. This work was supported by the National Basic Research Program of China (2011CB910500 and 2010CB833706 to SFS, and 2009CB118500 and 2015CB150100 to JZ) and the National Natural Science Foundation of China (31230016 and 31370717 to SFS).
Author information
Authors and Affiliations
Corresponding authors
Additional information
( Supplementary information is linked to the online version of the paper on the Cell Research website.)
Supplementary information
Supplementary information, Figure S1
Structural elements and available crystal structures of subunits in the PBS from Anabaena sp. strain PCC 7120. (PDF 182 kb)
Supplementary information, Figure S2
Sample preparation of intact PBSs from Anabaena sp. strain PCC 7120. (PDF 125 kb)
Supplementary information, Figure S3
Negative staining of intact PBS from Anabaena sp. strain PCC 7120. (PDF 152 kb)
Supplementary information, Figure S4
Coordinate system building and the orientation of each cylinder. (PDF 110 kb)
Supplementary information, Figure S5
Association of PSII to the PBS. (PDF 93 kb)
Supplementary information, Figure S6
Comparison of hexamers. (PDF 127 kb)
Supplementary information, Figure S7
Molecular reconstitution and purification of LRC mutated PBSs. (PDF 431 kb)
Supplementary information, Figure S8
Pigment distribution and energy transfer pathway. (PDF 181 kb)
Supplementary information, Figure S9
Two models of PSI in the complex PBS-PSII-PSI. (PDF 270 kb)
Supplementary information, Table S1
Cross-correlation coefficient (CCC) of crystal structures and the EM density map. (PDF 127 kb)
Supplementary information, Table S2
Primers used in this study. (PDF 130 kb)
Supplementary information, Movie S1
Structures of the intact PBS and the PBS-PSII complex. (MP4 8563 kb)
Rights and permissions
About this article
Cite this article
Chang, L., Liu, X., Li, Y. et al. Structural organization of an intact phycobilisome and its association with photosystem II. Cell Res 25, 726–737 (2015). https://doi.org/10.1038/cr.2015.59
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/cr.2015.59
Keywords
This article is cited by
-
Relationship between non-photochemical quenching efficiency and the energy transfer rate from phycobilisomes to photosystem II
Photosynthesis Research (2024)
-
Structural comparison of allophycocyanin variants reveals the molecular basis for their spectral differences
Photosynthesis Research (2023)
-
The organization of the phycobilisome-photosystem I supercomplex depends on the ratio between two different phycobilisome linker proteins
Photochemical & Photobiological Sciences (2023)
-
Allophycocyanin A is a carbon dioxide receptor in the cyanobacterial phycobilisome
Nature Communications (2022)
-
Structures of a phycobilisome in light-harvesting and photoprotected states
Nature (2022)