Abstract
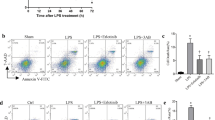
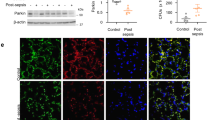
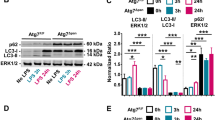
Inappropriate inflammation responses contribute to mortality during sepsis. Through Toll-like receptors (TLRs), reactive oxygen species (ROS) produced by NADPH oxidase could modulate the inflammation responses. Parkinson disease (autosomal recessive, early onset) 7 (Park7) has a cytoprotective role by eliminating ROS. However, whether Park7 could modulate inflammation responses and mortality in sepsis is unclear. Here, we show that, compared with wild-type mice, Park7−/− mice had significantly increased mortality and bacterial burdens in sepsis model along with markedly decreased systemic and local inflammation, and drastically impaired macrophage phagocytosis and bacterial killing abilities. Surprisingly, LPS and phorbol-12-myristate-13-acetate stimulation failed to induce ROS and proinflammatory cytokine production in Park7−/− macrophages and Park7-deficient RAW264.7 cells. Through its C-terminus, Park7 binds to p47phox, a subunit of the NADPH oxidase, to promote NADPH oxidase-dependent production of ROS. Restoration of Park7 expression rescues ROS production and improves survival in LPS-induced sepsis. Together, our study shows that Park7 has a protective role against sepsis by controlling macrophage activation, NADPH oxidase activation and inflammation responses.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Hotchkiss RS, Opal S . Immunotherapy for sepsis — a new approach against an ancient foe. N Engl J Med 2010; 363:87–89.
Angus DC, van der Poll T . Severe sepsis and septic shock. N Engl J Med 2013; 369:840–851.
Huber-Lang M, Barratt-Due A, Pischke SE, et al. Double blockade of CD14 and complement C5 abolishes the cytokine storm and improves morbidity and survival in polymicrobial sepsis in mice. J Immunol 2014; 192:5324–5331.
Boomer JS, To K, Chang KC, et al. Immunosuppression in patients who die of sepsis and multiple organ failure. JAMA 2011; 306:2594–2605.
Meisel C, Schefold JC, Pschowski R, et al. Granulocyte-macrophage colony-stimulating factor to reverse sepsis-associated immunosuppression: a double-blind, randomized, placebo-controlled multicenter trial. Am J Respir Crit Care Med 2009; 180:640–648.
Schefold JC, Hasper D, Volk HD, Reinke P . Sepsis: time has come to focus on the later stages. Med hypotheses 2008; 71:203–208.
Kanayama A, Miyamoto Y . Apoptosis triggered by phagocytosis-related oxidative stress through FLIPS down-regulation and JNK activation. J Leukoc Biol 2007; 82:1344–1352.
West AP, Brodsky IE, Rahner C, et al. TLR signalling augments macrophage bactericidal activity through mitochondrial ROS. Nature 2011; 472:476–480.
McCubrey JA, Lahair MM, Franklin RA . Reactive oxygen species-induced activation of the MAP kinase signaling pathways. Antioxid Redox Signal 2006; 8:1775–1789.
Takada Y, Mukhopadhyay A, Kundu GC, Mahabeleshwar GH, Singh S, Aggarwal BB . Hydrogen peroxide activates NF-kappa B through tyrosine phosphorylation of I kappa B alpha and serine phosphorylation of p65: evidence for the involvement of I kappa B alpha kinase and Syk protein-tyrosine kinase. J Biol Chem 2003; 278:24233–24241.
Bulua AC, Simon A, Maddipati R, et al. Mitochondrial reactive oxygen species promote production of proinflammatory cytokines and are elevated in TNFR1-associated periodic syndrome (TRAPS). J Exp Med 2011; 208:519–533.
Kong X, Thimmulappa R, Kombairaju P, Biswal S . NADPH oxidase-dependent reactive oxygen species mediate amplified TLR4 signaling and sepsis-induced mortality in Nrf2-deficient mice. J Immunol 2010; 185:569–577.
Hong Z, Shi M, Chung KA, et al. DJ-1 and alpha-synuclein in human cerebrospinal fluid as biomarkers of Parkinson's disease. Brain 2010; 133:713–726.
Yokota T, Sugawara K, Ito K, Takahashi R, Ariga H, Mizusawa H . Down regulation of DJ-1 enhances cell death by oxidative stress, ER stress, and proteasome inhibition. Biochem Biophy Res Commun 2003; 312:1342–1348.
Martinat C, Shendelman S, Jonason A, et al. Sensitivity to oxidative stress in DJ-1-deficient dopamine neurons: an ES- derived cell model of primary Parkinsonism. PLoS Biol 2004; 2:e327.
Kong X, Thimmulappa R, Craciun F, et al. Enhancing Nrf2 pathway by disruption of Keap1 in myeloid leukocytes protects against sepsis. Am J Respir Crit Care Med 2011; 184:928–938.
Calandra T, Baumgartner JD, Grau GE, et al. Prognostic values of tumor necrosis factor/cachectin,interleukin-1, interferon-alpha, and interferon-gamma in the serum of patients with septic shock. Swiss-Dutch J5 Immunoglobulin Study Group. J Infect Dis 1990; 161:982–987.
Damas P, Ledoux D, Nys M, Vrindts Y, De Groote D, Franchimont P, Lamy M . Cytokine serum level during severe sepsis in human IL-6 as a marker of severity. Ann Surg 1992; 215:356–362.
Underhill DM, Ozinsky A . Phagocytosis of microbes: complexity in action. Annu Rev Immunol 2002; 20:825–852.
Meador BM, Krzyszton CP, Johnson RW, Huey KA . Effects of IL-10 and age on IL-6, IL-1beta, and TNF-alpha responses in mouse skeletal and cardiac muscle to an acute inflammatory insult. J Appl Physiol (1985) 2008; 104:991–997.
Lee CC, Avalos AM, Ploegh HL . Accessory molecules for Toll-like receptors and their function. Nat Rev Immunol 2012; 12:168–179.
Gallego C, Golenbock D, Gomez MA, Saravia NG . Toll-like receptors participate in macrophage activation and intracellular control of Leishmania (Viannia) panamensis. Infect Immun 2011; 79:2871–2879.
Akira S, Takeda K . Toll-like receptor signaling. Nat Rev Immunol 2004; 4:499–511.
Karin M . The regulation of AP-1 activity by mitogen-activated protein kinases. J Biol Chem 1995; 270:16483–16486.
Pahl HL . Activators and target genes of Rel/NF-kappaB transcription factors. Oncogene 1999; 18:6853–6866.
Wu CC, Hsu SC, Shih HM, Lai MZ . Nuclear factor of activated T cells c is a target of p38 mitogen-activated protein kinase in T cells. Mol Cell Biol 2003; 23:6442–6454.
Warn PA, Brampton MW, Sharp A, et al. Infrared body temperature measurement of mice as an early predictor of death in experimental fungal infections. Lab Anim 2003; 37:126–131.
Nakahira K, Kim HP, Geng XH, et al. Carbon monoxide differentially inhibits TLR signaling pathways by regulating ROS-induced trafficking of TLRs to lipid rafts. J Exp Med 2006; 203:2377–2389.
Kensler TW, Wakabayashi N, Biswal S . Cell survival responses to environmental stresses via the Keap1-Nrf2-ARE pathway. Annu Rev Pharmacol Toxicol 2007; 47:89–116.
Foller M, Harris IS, Elia A, et al. Functional significance of glutamate-cysteine ligase modifier for erythrocyte survival in vitro and in vivo. Cell Death Differ 2013; 20:1350–1358.
Im JY, Lee KW, Woo JM, Junn E, Mouradian MM . DJ-1 induces thioredoxin 1 expression through the Nrf2 pathway. Hum Mol Genet 2012; 21:3013–3024.
Fernandes DC, Wosniak J, Pescatore LA, et al. Analysis of DHE-derived oxidation products by HPLC in the assessment of superoxide production and NADPH oxidase activity in vascular systems. Am J Physiol Cell Physiol 2007; 292:C413–C422.
Kato I, Maita H, Takahashi-Niki K, et al. Oxidized DJ-1 inhibits p53 by sequestering p53 from promoters in a DNA-binding affinity-dependent manner. Mol Cell Biol 2013; 33:340–359.
Kim YC, Kitaura H, Taira T, Iguchi-Ariga SM, Ariga H . Oxidation of DJ-1-dependent cell transformation through direct binding of DJ-1 to PTEN. Int J Oncol 2009; 35:1331–1341.
Yellaboina S, Tasneem A, Zaykin DV, Raghavachari B, Jothi R . DOMINE: a comprehensive collection of known and predicted domain-domain interactions. Nucleic Acids Res 2011; 39:D730–D735.
Raghavachari B, Tasneem A, Przytycka TM, Jothi R . DOMINE: a database of protein domain interactions. Nucleic Acids Res 2008; 36:D656–D661.
Waak J, Weber SS, Gorner K, et al. Oxidizable residues mediating protein stability and cytoprotective interaction of DJ-1 with apoptosis signal-regulating kinase 1. J Biol Chem 2009; 284:14245–14257.
Lambeth JD . NOX enzymes and the biology of reactive oxygen. Nat Rev Immunol 2004; 4:181–189.
Anderson KE, Chessa TA, Davidson K, et al. PtdIns3P and Rac direct the assembly of the NADPH oxidase on a novel, pre-phagosomal compartment during FcR-mediated phagocytosis in primary mouse neutrophils. Blood 2010; 116:4978–4989.
Kong XN, Yan HX, Chen L, et al. LPS-induced down-regulation of signal regulatory protein {alpha} contributes to innate immune activation in macrophages. J Exp Med 2007; 204:2719–2731.
Alazawi W, Heath H, Waters JA, et al. Stat2 loss leads to cytokine-independent, cell-mediated lethality in LPS-induced sepsis. Proc Natl Acad Sci USA 2013; 110:8656–8661.
Vasseur S, Afzal S, Tomasini R, et al. Consequences of DJ-1 upregulation following p53 loss and cell transformation. Oncogene 2012; 31:664–670.
Hassan F, Islam S, Mu MM, et al. Lipopolysaccharide prevents doxorubicin-induced apoptosis in RAW 264.7 macrophage cells by inhibiting p53 activation. Mol Cancer Res 2005; 3:373–379.
Jackson SH, Gallin JI, Holland SM . The p47phox mouse knock-out model of chronic granulomatous disease. J Exp Med 1995; 182:751–758.
Kim KS, Kim J S, Park J Y, et al. DJ-1 associates with lipid rafts by palmitoylation and regulates lipid rafts-dependent endocytosis in astrocytes. Hum Mol Genet, 2013. 22:4805–4817.
Choi YK, Elaine D, Kwon Y G, et al. Regulation of ROS production and vascular function by carbon monoxide. Oxid Med Cell Longev 2012; 2012:794237.
Rada B, Gardina P, Myers TG, Leto TL . Reactive oxygen species mediate inflammatory cytokine release and EGFR-dependent mucin secretion in airway epithelial cells exposed to Pseudomonas pyocyanin. Mucosal immunol 2011; 4:158–171.
Annane D, Bellissant E, Cavaillon JM . Septic shock. Lancet 2005; 365:63–78.
Spooner CE, Markowitz NP, Saravolatz LD . The role of tumor necrosis factor in sepsis. Clin Immunol Immunopathol 1992; 62:S11–S17.
Suffredini AF, Munford RS . Novel therapies for septic shock over the past 4 decades. JAMA 2011; 306:194–199.
Acknowledgements
We thank Dr Liwei Dong (The Second Military Medical University) for the luciferase reporter vectors, Dr Li Shen (Tongji University School of Medicine) for the Pseudomonas aeruginosa and Dr Dechun Feng (National Institute on Alcohol Abuse and Alcoholism) for helpful comments. This work was supported by the Ministry of Science and Technology of China (2012CB966800 and 2013CB945600) to WQG, the National Natural Science Foundation of China (81130038 and 81372189 to WQG, and 31300742 to XK), the Science and Technology Commission of Shanghai Municipality (Pujiang program), the Shanghai Health Bureau Key Disciplines and Specialties Foundation, the Shanghai Education Committee Key Discipline and Specialties Foundation (J50208) and KC Wong foundation and funds to WQG, and the Shanghai Education Committee (Eastern Scholar Program) to XK.
Author information
Authors and Affiliations
Corresponding authors
Additional information
( Supplementary information is linked to the online version of the paper on the Cell Research website.)
Supplementary information
Supplementary information, Figure S1
(A) Western blot of Park7 expression in indicated cells and tissues of WT and Park7-KO mice. (PDF 248 kb)
Supplementary information, Figure S2
Depletion of Park7 impairs LPS induced proinflammatory cytokine production in RAW264.7 cells. (PDF 109 kb)
Supplementary information, Figure S3
Phosphorylation of IKBa and IRF3 by western blotting, at indicated time points, in NT and KD RAW264.7 cells after LPS. (PDF 23 kb)
Supplementary information, Figure S4
Quantification of mRNA levels, at indicated time points, of ROS scavenger genes, Nfe2l2, Gclm and Trx1, in peritoneal macrophages isolated from WT and Park7−/− by qPCR after LPS. (PDF 17 kb)
Supplementary information, Figure S5
(A) Quantification of Rac2 level after Rac2 siRNA treatment. (PDF 27 kb)
Supplementary information, Figure S6
Doxycycline induced Park7 expression. (PDF 30 kb)
Supplementary information, Figure S7
Impaired immune-repelling in macrophage-depleted C57BL/6 mice with RAW264.7 transfer. (PDF 574 kb)
Supplementary information, Figure S8
Cys106 oxidation in Park7. (PDF 22 kb)
Supplementary information, Data S1
Materials and Methods (PDF 221 kb)
Rights and permissions
About this article
Cite this article
Liu, W., Wu, H., Chen, L. et al. Park7 interacts with p47phox to direct NADPH oxidase-dependent ROS production and protect against sepsis. Cell Res 25, 691–706 (2015). https://doi.org/10.1038/cr.2015.63
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/cr.2015.63
Keywords
This article is cited by
-
A metabolite from commensal Candida albicans enhances the bactericidal activity of macrophages and protects against sepsis
Cellular & Molecular Immunology (2023)
-
Characterization of gene expression profiles in the mouse brain after 35 days of spaceflight mission
npj Microgravity (2022)
-
PARK7/DJ-1 promotes pyruvate dehydrogenase activity and maintains Treg homeostasis during ageing
Nature Metabolism (2022)
-
NADPH oxidase in brain injury and neurodegenerative disorders
Molecular Neurodegeneration (2017)
-
NADPH oxidases in Parkinson’s disease: a systematic review
Molecular Neurodegeneration (2017)