Abstract
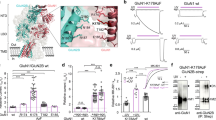
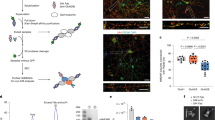
The N-methyl-D-aspartate receptor (NMDAR) in adult forebrain is a heterotetramer mainly composed of two GluN1 subunits and two GluN2A and/or GluN2B subunits. The synaptic expression and relative numbers of GluN2A- and GluN2B-containing NMDARs play critical roles in controlling Ca2+-dependent signaling and synaptic plasticity. Previous studies have suggested that the synaptic trafficking of NMDAR subtypes is differentially regulated, but the precise molecular mechanism is not yet clear. In this study, we demonstrated that Bip, an endoplasmic reticulum (ER) chaperone, selectively interacted with GluN2A and mediated the neuronal activity-induced assembly and synaptic incorporation of the GluN2A-containing NMDAR from dendritic ER. Furthermore, the GluN2A-specific synaptic trafficking was effectively disrupted by peptides interrupting the interaction between Bip and GluN2A. Interestingly, fear conditioning in mice was disrupted by intraperitoneal injection of the interfering peptide before training. In summary, we have uncovered a novel mechanism for the activity-dependent supply of synaptic GluN2A-containing NMDARs, and demonstrated its relevance to memory formation.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Cull-Candy S, Brickley S, Farrant M . NMDA receptor subunits: diversity, development and disease. Curr Opin Neurobiol 2001; 11:327–335.
Yashiro K, Philpot BD . Regulation of NMDA receptor subunit expression and its implications for LTD, LTP, and metaplasticity. Neuropharmacology 2008; 55:1081–1094.
de Armentia ML, Sah P . Development and subunit composition of synaptic NMDA receptors in the amygdala: NR2B synapses in the adult central amygdala. J Neurosci 2003; 23:6876–6883.
Nase G, Weishaupt J, Stern P, Singer W, Monyer H . Genetic and epigenetic regulation of NMDA receptor expression in the rat visual cortex. Eur J Neurosci 1999; 11:4320–4326.
Liu XB, Murray KD, Jones EG . Switching of NMDA receptor 2A and 2B subunits at thalamic and cortical synapses during early postnatal development. J Neurosci 2004; 24:8885–8895.
Philpot BD, Espinosa JS, Bear MF . Evidence for altered NMDA receptor function as a basis for metaplasticity in visual cortex. J Neurosci 2003; 23:5583–5588.
Sawtell NB, Frenkel MY, Philpot BD, Nakazawa K, Tonegawa S, Bear MF . NMDA receptor-dependent ocular dominance plasticity in adult visual cortex. Neuron 2003; 38:977–985.
Yashiro K, Corlew R, Philpot BD . Visual deprivation modifies both presynaptic glutamate release and the composition of perisynaptic/extrasynaptic NMDA receptors in adult visual cortex. J Neurosci 2005; 25:11684–11692.
Philpot BD, Sekhar AK, Shouval HZ, Bear MF . Visual experience and deprivation bidirectionally modify the composition and function of NMDA receptors in visual cortex. Neuron 2001; 29:157–169.
Philpot BD, Cho KKA, Bear MF . Obligatory role of NR2A for metaplasticity in visual cortex. Neuron 2007; 53:495–502.
Quinlan EM, Lebel D, Brosh I, Barkai E . A molecular mechanism for stabilization of learning-induced synaptic modifications. Neuron; 41:185–192.
Kopp C, Longordo F, Lüthi A . Experience-dependent changes in NMDA receptor composition at mature central synapses. Neuropharmacology 2007; 53:1–9.
Chen B-S, Roche KW . Regulation of NMDA receptors by phosphorylation. Neuropharmacology 2007; 53:362–368.
Chung HJ, Huang YH, Lau LF, Huganir RL . Regulation of the NMDA receptor complex and trafficking by activity-dependent phosphorylation of the NR2B subunit PDZ ligand. J Neurosci 2004; 24:10248–10259.
Tovar KR, Westbrook GL . Mobile NMDA receptors at hippocampal synapses. Neuron 2002; 34:253–264.
Groc L, Heine M, Cousins SL, et al. NMDA receptor surface mobility depends on NR2A-2B subunits. Proc Natl Acad Sci USA 2006; 103:18769–18774.
Prybylowski K, Chang K, Sans N, Kan L, Vicini S, Wenthold RJ . The synaptic localization of NR2B-containing NMDA receptors is controlled by interactions with PDZ proteins and AP-2. Neuron 2005; 47:845–857.
Vissel B, Krupp JJ, Heinemann SF, Westbrook GL . A use-dependent tyrosine dephosphorylation of NMDA receptors is independent of ion flux. Nat Neurosci 2001; 4:587–596.
Horton AC, Ehlers MD . Dual modes of endoplasmic reticulum-to-Golgi transport in dendrites revealed by live-cell imaging. J Neurosci 2003; 23:6188–6199.
Ramirez OA, Couve A . The endoplasmic reticulum and protein trafficking in dendrites and axons. Trends Cell Biol 2011; 21:219–227.
Guillaud L, Setou M, Hirokawa N . KIF17 dynamics and regulation of NR2B trafficking in hippocampal neurons. J Neurosci 2003; 23:131–140.
Setou M, Nakagawa T, Seog DH, Hirokawa N . Kinesin superfamily motor protein KIF17 and mLin-10 in NMDA receptor–containing vesicle transport. Science 2000; 288:1796–1802.
Jeyifous O, Lwaites C, Specht CG, et al. SAP97 and CASK mediate sorting of NMDA receptors through a previously unknown secretory pathway. Nat Neurosci 2009; 12:1011–U1081.
Mauceri D, Gardoni F, Marcello E, Di Luca M . Dual role of CaMKII-dependent SAP97 phosphorylation in mediating trafficking and insertion of NMDA receptor subunit NR2A. J Neurochem 2007; 100:1032–1046.
Swanger SA, He YA, Richter JD, Bassell GJ . Dendritic GluN2A synthesis mediates activity-induced NMDA receptor insertion. J Neurosci 2013; 33:8898–8908.
Udagawa T, Swanger SA, Takeuchi K, et al. Bidirectional control of mRNA translation and synaptic plasticity by the cytoplasmic polyadenylation complex. Mol Cell 2012; 47:253–266.
Qiu S, Zhang XM, Cao JY, et al. An endoplasmic reticulum retention signal located in the extracellular amino-terminal domain of the NR2A subunit of N-Methyl-D-aspartate receptors. J Biol Chem 2009; 284:20285–20298.
Dani A, Huang B, Bergan J, Dulac C, Zhuang X . Superresolution imaging of chemical synapses in the brain. Neuron 2010; 68:843–856.
Hayashi Y, Shi SH, Esteban JA, Piccini A, Poncer JC, Malinow R . Driving AMPA receptors into synapses by LTP and CaMKII: requirement for GluR1 and PDZ domain interaction. Science 2000; 287:2262–2267.
Lu WY, Man HY, Ju W, Trimble WS, MacDonald JF, Wang YT . Activation of synaptic NMDA receptors induces membrane insertion of new AMPA receptors and LTP in cultured hippocampal neurons. Neuron 2001; 29:243–254.
Ori-McKenney Kassandra M, Jan LY, Jan YN . Golgi outposts shape dendrite morphology by functioning as sites of acentrosomal microtubule nucleation in neurons. Neuron 2012; 76:921–930.
Klausner RD, Donaldson JG, Lippincott-Schwartz J . Brefeldin A: insights into the control of membrane traffic and organelle structure. J Cell Biol 1992; 116:1071–1080.
Hirokawa N, Niwa S, Tanaka Y . Molecular motors in neurons: transport mechanisms and roles in brain function, development, and disease. Neuron 2010; 68:610–638.
Torre ER, Steward O . Protein synthesis within dendrites: Glycosylation of newly synthesized proteins in dendrites of hippocampal neurons in culture. J Neurosci 1996; 16:5967–5978.
Horton AC, Racz B, Monson EE, Lin AL, Weinberg RJ, Ehlers MD . Polarized secretory trafficking directs cargo for asymmetric dendrite growth and morphogenesis. Neuron 2005; 48:757–771.
Pierce JP, Mayer T, McCarthy JB . Evidence for a satellite secretory pathway in neuronal dendritic spines. Curr Biol 2001; 11:351–355.
Biermann B, Ivankova-Susankova K, Bradaia A, et al. The sushi domains of GABAB receptors function as axonal targeting signals. J Neurosci 2010; 30:1385–1394.
Ramirez OA, Vidal RL, Tello JA, et al. Dendritic assembly of heteromeric γ-aminobutyric acid type B receptor subunits in hippocampal neurons. J Biol Chem 2009; 284:13077–13085.
Hasdemir B, Fitzgerald DJ, Prior IA, Tepikin AV, Burgoyne RD . Traffic of Kv4 K+ channels mediated by KChIP1 is via a novel post-ER vesicular pathway. J Cell Biol 2005; 171:459–469.
Hu T, Kao C-Y, Hudson RT, Chen A, Draper RK . Inhibition of secretion by 1,3-cyclohexanebis(methylamine), a dibasic compound that interferes with coatomer function. Mol Biol Cell 1999; 10:921–933.
Bellone C, Nicoll RA . Rapid bidirectional switching of synaptic NMDA receptors. Neuron 2007; 55:779–785.
Matta JA, Ashby MC, Sanz-Clemente A, Roche KW, Isaac JTR . mGluR5 and NMDA receptors drive the experience- and activity-dependent NMDA receptor NR2B to NR2A subunit switch. Neuron 2011; 70:339–351.
Kennedy MJ, Ehlers MD . Organelles and trafficking machinery for postsynaptic plasticity. Annu Rev Neurosci 2006; 29:325–362.
Correia SS, Bassani S, Brown TC, et al. Motor protein-dependent transport of AMPA receptors into spines during long-term potentiation. Nat Neurosci 2008; 11:457–466.
Zakharenko S, Chang S, O'Donoghue M, Popov SV . Neurotransmitter secretion along growing nerve processes: comparison with synaptic vesicle exocytosis. J Cell Biol 1999; 144:507–518.
Tanaka T, Serneo FF, Higgins C, Gambello MJ, Wynshaw-Boris A, Gleeson JG . Lis1 and doublecortin function with dynein to mediate coupling of the nucleus to the centrosome in neuronal migration. J Cell Biol 2004; 165:709–721.
Sharp DJ, Yu W, Ferhat L, Kuriyama R, Rueger DC, Baas PW . Identification of a microtubule-associated motor protein essential for dendritic differentiation. J Cell Biol 1997; 138:833–843.
Xu Z, Chen RQ, Gu QH, et al. Metaplastic regulation of long-term potentiation/long-term depression threshold by activity-dependent changes of NR2A/NR2B ratio. J Neurosci 2009; 29:8764–8773.
Sanz-Clemente A, Matta JA, Isaac JTR, Roche KW . Casein kinase 2 regulates the NR2 subunit composition of synaptic NMDA receptors. Neuron 2010; 67:984–996.
Chung HJ, Huang YH, Lau L-F, Huganir RL . Regulation of the NMDA receptor complex and trafficking by activity-dependent phosphorylation of the NR2B subunit PDZ ligand. J Neurosci 2004; 24:10248–10259.
Zhang S, Edelmann L, Liu J, Crandall JE, Morabito MA . Cdk5 Regulates the phosphorylation of tyrosine 1472 NR2B and the surface expression of NMDA receptors. J Neurosci 2008; 28:415–424.
Tovar KR, Westbrook GL . Mobile NMDA receptors at hippocampal synapses. Neuron 2002; 34:255–264.
Bramham CR, Wells DG . Dendritic mRNA: transport, translation and function. Nat Rev Neurosci 2007; 8:776–789.
Hanus C, Ehlers MD . Secretory outposts for the local processing of membrane cargo in neuronal dendrites. Traffic 2008; 9:1437–1445.
Cui-Wang T, Hanus C, Cui T, et al. Local zones of endoplasmic reticulum complexity confine cargo in neuronal dendrites. Cell 2012; 148:309–321.
Park M, Penick EC, Edwards JG, Kauer JA, Ehlers MD . Recycling endosomes supply AMPA receptors for LTP. Science 2004; 305:1972–1975.
Ellgaard L, Helenius A . Quality control in the endoplasmic reticulum. Nat Rev Mol Cell Biol 2003; 4:181–191.
Otero JH, Lizák B, Hendershot LM . Life and death of a BiP substrate. Semin Cell Dev Biol 2010; 21:472–478.
Lee YK, Brewer JW, Hellman R, Hendershot LM . BiP and immunoglobulin light chain cooperate to control the folding of heavy chain and ensure the fidelity of immunoglobulin assembly. Mol Biol Cell 1999; 10:2209–2219.
Vanhove M, Usherwood YK, Hendershot LM . Unassembled Ig heavy chains do not cycle from BiP in vivo but require light chains to trigger their release. Immunity 2001; 15:105–114.
Pabba M, Wong AY, Ahlskog N, et al. NMDA receptors are upregulated and trafficked to the plasma membrane after sigma-1 receptor activation in the rat hippocampus. J Neurosci 2014; 34:11325–11338.
Su TP, Hayashi T, Maurice T, Buch S, Ruoho AE . The sigma-1 receptor chaperone as an inter-organelle signaling modulator. Trends Pharmacol Sci 2010; 31:557–566.
Ortega-Roldan JL, Ossa F, Schnell JR . Characterization of the human sigma-1 receptor chaperone domain structure and binding immunoglobulin protein (BiP) interactions. J Biol Chem 2013; 288:21448–21457.
Peng Y, Zhao J, Gu QH, et al. Distinct trafficking and expression mechanisms underlie LTP and LTD of NMDA receptor-mediated synaptic responses. Hippocampus 2010; 20:646–658.
Grosshans DR, Clayton DA, Coultrap SJ, Browning MD . LTP leads to rapid surface expression of NMDA but not AMPA receptors in adult rat CA1. Nat Neurosci 2002; 5:27–33.
Hunt DL, Castillo PE . Synaptic plasticity of NMDA receptors: mechanisms and functional implications. Curr Opin Neurobiol 2012; 22:496–508.
Thiagarajan TC, Lindskog M, Tsien RW . Adaptation to synaptic inactivity in hippocampal neurons. Neuron 2005; 47:725–737.
Abraham WC . Metaplasticity: tuning synapses and networks for plasticity. Nat Rev Neurosci 2008; 9:387–387.
Kiyama Y, Manabe T, Sakimura K, Kawakami F, Mori H, Mishina M . Increased thresholds for long-term potentiation and contextual learning in mice lacking the NMDA-type glutamate receptor ɛ1 subunit. J Neurosci 1998; 18:6704–6712.
von Engelhardt J, Doganci B, Jensen V, et al. Contribution of hippocampal and extra-hippocampal NR2B-containing NMDA receptors to performance on spatial learning tasks. Neuron 2008; 60:846–860.
Miserendino MJD, Sananes CB, Melia KR, Davis M . Blocking of acquisition but not expression of conditioned fear-potentiated startle by NMDA antagonists in amygdala. Nature 1990; 345:716–718.
Chenard BL, Menniti FS . Antagonists selective for NMDA receptors containing the NR2B subunit. Curr Pharm Des 1999; 5:381–404.
Rodrigues SM, Schafe GE, LeDoux JE . Intra-amygdala blockade of the NR2B subunit of the NMDA receptor disrupts the acquisition but not the expression of fear conditioning. J Neurosci 2001; 21:6889–6896.
Qiu S, Zhang XM, Cao JY, et al. An endoplasmic reticulum retention signal located in the extracellular amino-terminal domain of the NR2A subunit of N-methyl-D-aspartate receptors. J Biol Chem 2009; 284:20285–20298.
Qiu S, Hua YL, Yang F, Chen YZ, Luo JH . Subunit assembly of N-methyl-D-aspartate receptors analyzed by fluorescence resonance energy transfer. J Biol Chem 2005; 280:24923–24930.
Luo JH, Fu ZY, Losi G, et al. Functional expression of distinct NMDA channel subunits tagged with green fluorescent protein in hippocampal neurons in culture. Neuropharmacology 2002; 42:306–318.
Acknowledgements
This work was supported by grants from the National Basic Research Program of China (2010CB912002), the National Natural Science Foundation of China (91232303, 81221003, 81371302 and 31460258) and the Fundamental Research Funds for the Central Universities of China. We thank Dr Guoqiang Bi from the University of Science and Technology of China for his help with STORM and other cell imaging experiments.
Author information
Authors and Affiliations
Corresponding authors
Additional information
( Supplementary information is linked to the online version of the paper on the Cell Research website.)
Supplementary information
Supplementary information, Figure S1
(A) BFA or CBM applying disrupts ER-to-Golgi transport in dendrites. (PDF 32 kb)
Supplementary information, Figure S2
(A) CHX was applied simultaneous or 0.5 h before depolarization (KCl) or cLTP stimulation. (PDF 37 kb)
Supplementary information, Figure S3
(A,B) The orginal gel of Figure 7C. (PDF 54 kb)
Supplementary information, Figure S4
(A) Quantification of the effect of disrupting peptides on surface expression of GluN2A-containing NMDARs induced by neuronal activity. (PDF 2898 kb)
Supplementary information, Movie S1
Transport of mitochondria along microtubules was inhibited by nocodazole Mitochondria were labeled by mitotracker in living neurons and tracked under a fluorescent confocal microscope under basal condition. (MOV 630 kb)
Supplementary information, Movie S2
Transport of mitochondria along microtubules was inhibited by nocodazole Mitochondria were labeled by mitotracker in living neurons and tracked under a fluorescent confocal microscope under nocodazole treatment conditions. (MOV 656 kb)
Rights and permissions
About this article
Cite this article
Zhang, Xm., Yan, Xy., Zhang, B. et al. Activity-induced synaptic delivery of the GluN2A-containing NMDA receptor is dependent on endoplasmic reticulum chaperone Bip and involved in fear memory. Cell Res 25, 818–836 (2015). https://doi.org/10.1038/cr.2015.75
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/cr.2015.75
Keywords
This article is cited by
-
The Regulation of GluN2A by Endogenous and Exogenous Regulators in the Central Nervous System
Cellular and Molecular Neurobiology (2017)