Abstract
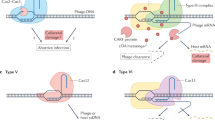
Clustered regularly interspaced short palindromic repeats (CRISPR)-CRISPR-associated (Cas) systems in bacteria and archaea provide adaptive immunity against invading foreign nucleic acids. Previous studies suggest that certain bacteria employ their Type II CRISPR-Cas systems to target their own genes, thus evading host immunity. However, whether other CRISPR-Cas systems have similar functions during bacterial invasion of host cells remains unknown. Here we identify a novel role for Type I CRISPR-Cas systems in evading host defenses in Pseudomonas aeruginosa strain UCBPP-PA14. The Type I CRISPR-Cas system of PA14 targets the mRNA of the bacterial quorum-sensing regulator LasR to dampen the recognition by toll-like receptor 4, thus diminishing the pro-inflammatory responses of the host in cell and mouse models. Mechanistically, this nuclease-mediated RNA degradation requires a “5′-GGN-3′” recognition motif in the target mRNA, and HD and DExD/H domains in Cas3 of the Type I CRISPR-Cas system. As LasR and Type I CRISPR-Cas systems are ubiquitously present in bacteria, our findings elucidate an important common mechanism underlying bacterial virulence.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Carte J, Christopher RT, Smith JT, et al. The three major types of CRISPR-Cas systems function independently in CRISPR RNA biogenesis in Streptococcus thermophilus. Mol Microbiol 2014; 93:98–112.
Wiedenheft B, Sternberg SH, Doudna JA . RNA-guided genetic silencing systems in bacteria and archaea. Nature 2012; 482:331–338.
Makarova KS, Wolf YI, Alkhnbashi OS, et al. An updated evolutionary classification of CRISPR-Cas systems. Nat Rev Microbiol 2015; 13:722–736.
Shmakov S, Abudayyeh OO, Makarova KS, et al. Discovery and functional characterization of diverse class 2 CRISPR-Cas systems. Mol Cell 2015; 60:385–397.
Westra ER, Buckling A, Fineran PC . CRISPR-Cas systems: beyond adaptive immunity. Nat Rev Microbiol 2014; 12:317–326.
Sampson TR, Saroj SD, Llewellyn AC, Tzeng YL, Weiss DS . A CRISPR/Cas system mediates bacterial innate immune evasion and virulence. Nature 2013; 497:254–257.
Louwen R, Horst-Kreft D, de Boer AG, et al. A novel link between Campylobacter jejuni bacteriophage defence, virulence and Guillain-Barre syndrome. Eur J Clin Microbiol Infect Dis 2013; 32:207–226.
Aklujkar M, Lovley DR . Interference with histidyl-tRNA synthetase by a CRISPR spacer sequence as a factor in the evolution of Pelobacter carbinolicus. BMC Evol Biol 2010; 10:230.
Jorth P, Whiteley M . An evolutionary link between natural transformation and CRISPR adaptive immunity. MBio 2012; 3. pii:e00309–e00312.
Lyczak JB, Cannon CL, Pier GB . Establishment of Pseudomonas aeruginosa infection: lessons from a versatile opportunist. Microbes Infect 2000; 2:1051–1060.
Rahme LG, Stevens EJ, Wolfort SF, Shao J, Tompkins RG, Ausubel FM . Common virulence factors for bacterial pathogenicity in plants and animals. Science 1995; 268:1899–1902.
Tan MW, Rahme LG, Sternberg JA, Tompkins RG, Ausubel FM . Pseudomonas aeruginosa killing of Caenorhabditis elegans used to identify P. aeruginosa virulence factors. Proc Natl Acad Sci USA 1999; 96:2408–2413.
Zegans ME, Wagner JC, Cady KC, Murphy DM, Hammond JH, O'Toole GA . Interaction between bacteriophage DMS3 and host CRISPR region inhibits group behaviors of Pseudomonas aeruginosa. J Bacteriol 2009; 191:210–219.
Kooistra O, Bedoux G, Brecker L, et al. Structure of a highly phosphorylated lipopolysaccharide core in the Delta algC mutants derived from Pseudomonas aeruginosa wild-type strains PAO1 (serogroup O5) and PAC1R (serogroup O3). Carbohydr Res 2003; 338:2667–2677.
Cady KC, O'Toole GA . Non-identity-mediated CRISPR-bacteriophage interaction mediated via the Csy and Cas3 proteins. J Bacteriol 2011; 193:3433–3445.
Hauser AR . The type III secretion system of Pseudomonas aeruginosa: infection by injection. Nat Rev Microbiol 2009; 7:654–665.
Sawa T, Shimizu M, Moriyama K, Wiener-Kronish JP . Association between Pseudomonas aeruginosa type III secretion, antibiotic resistance, and clinical outcome: a review. Crit Care 2014; 18:668.
Zhao KL, Li Y, Yue BS, Wu M . Genes as early responders regulate quorum-sensing and control bacterial cooperation in Pseudomonas aeruginosa. PLoS One 2014; 9:e101887.
Cass SD, Haas KA, Stoll B, et al. The role of Cas8 in type I CRISPR interference. Biosci Rep 2015; 35. pii: e00197.
Bernstein JA, Khodursky AB, Lin PH, Lin-Chao S, Cohen SN . Global analysis of mRNA decay and abundance in Escherichia coli at single-gene resolution using two-color fluorescent DNA microarrays. Proc Natl Acad Sci USA 2002; 99:9697–9702.
Rollins MF, Schuman JT, Paulus K, Bukhari HS, Wiedenheft B . Mechanism of foreign DNA recognition by a CRISPR RNA-guided surveillance complex from Pseudomonas aeruginosa. Nucleic Acids Res 2015; 43:2216–2222.
Li R, Tan S, Yu M, Jundt MC, Zhang S, Wu M . Annexin A2 regulates autophagy in Pseudomonas aeruginosa infection through the Akt1-mTOR-ULK1/2 signaling pathway. J Immunol 2015; 195:3901–3911.
Mei SH, Haitsma JJ, Dos Santos CC, et al. Mesenchymal stem cells reduce inflammation while enhancing bacterial clearance and improving survival in sepsis. Am J Respir Crit Care Med 2010; 182:1047–1057.
Gao Y, Fang X, Tong Y, Liu Y, Zhang B . TLR4-mediated MyD88-dependent signaling pathway is activated by cerebral ischemia-reperfusion in cortex in mice. Biomed Pharmacother 2009; 63:442–450.
Choi H, Choi H, Han J, et al. IL-4 inhibits the melanogenesis of normal human melanocytes through the JAK2-STAT6 signaling pathway. J Invest Dermatol 2013; 133:528–536.
Zhou X, Li X, Ye Y, et al. MicroRNA-302b augments host defense to bacteria by regulating inflammatory responses via feedback to TLR/IRAK4 circuits. Nat Commun 2014; 5:3619.
Kiratisin P, Tucker KD, Passador L . LasR, a transcriptional activator of Pseudomonas aeruginosa virulence genes, functions as a multimer. J Bacteriol 2002; 184:4912–4919.
Staals RH, Zhu Y, Taylor DW, et al. RNA targeting by the type III-A CRISPR-Cas Csm complex of Thermus thermophilus. Mol Cell 2014; 56:518–530.
Gunderson FF, Cianciotto NP . The CRISPR-associated gene cas2 of Legionella pneumophila is required for intracellular infection of amoebae. MBio 2013; 4:e00074–e00013.
Luo ML, Mullis AS, Leenay RT, Beisel CL . Repurposing endogenous type I CRISPR-Cas systems for programmable gene repression. Nucleic Acids Res 2015; 43:674–681.
Sinkunas T, Gasiunas G, Fremaux C, Barrangou R, Horvath P, Siksnys V . Cas3 is a single-stranded DNA nuclease and ATP-dependent helicase in the CRISPR/Cas immune system. EMBO J 2011; 30:1335–1342.
Wiedenheft B, van Duijn E, Bultema JB, et al. RNA-guided complex from a bacterial immune system enhances target recognition through seed sequence interactions. Proc Natl Acad Sci USA 2011; 108:10092–10097.
Wiedenheft B, Zhou K, Jinek M, Coyle SM, Ma W, Doudna JA . Structural basis for DNase activity of a conserved protein implicated in CRISPR-mediated genome defense. Structure 2009; 17:904–912.
Weiss DS, Brotcke A, Henry T, Margolis JJ, Chan K, Monack DM . In vivo negative selection screen identifies genes required for Francisella virulence. Proc Natl Acad Sci USA 2007; 104:6037–6042.
Buchanan PJ, Ernst RK, Elborn JS, Schock B . Role of CFTR, Pseudomonas aeruginosa and toll-like receptors in cystic fibrosis lung inflammation. Biochem Soc Trans 2009; 37:863–867.
Epelman S, Stack D, Bell C, et al. Different domains of Pseudomonas aeruginosa exoenzyme S activate distinct TLRs. J Immunol 2004; 173:2031–2040.
van der Oost J, Jore MM, Westra ER, Lundgren M, Brouns SJ . CRISPR-based adaptive and heritable immunity in prokaryotes. Trends Biochem Sci 2009; 34:401–407.
Louwen R, Staals RH, Endtz HP, van Baarlen P, van der Oost J . The role of CRISPR-Cas systems in virulence of pathogenic bacteria. Microbiol Mol Biol Rev 2014; 78:74–88.
Stern A, Keren L, Wurtzel O, Amitai G, Sorek R . Self-targeting by CRISPR: gene regulation or autoimmunity? Trends Genet 2010; 26:335–340.
Fodah RA, Scott JB, Tam HH, et al. Correlation of Klebsiella pneumoniae comparative genetic analyses with virulence profiles in a murine respiratory disease model. PLoS One 2014; 9:e107394.
Hochstrasser ML, Taylor DW, Bhat P, et al. CasA mediates Cas3-catalyzed target degradation during CRISPR RNA-guided interference. Proc Natl Acad Sci USA 2014; 111:6618–6623.
Kannan S, Huang H, Seeger D, et al. Alveolar epithelial type II cells activate alveolar macrophages and mitigate P. aeruginosa infection. PLoS One 2009; 4:e4891.
Li G, Fox J, 3rd, Liu Z, et al. Lyn mitigates mouse airway remodeling by downregulating the TGF-beta3 isoform in house dust mite models. J Immunol 2013; 191:5359–5370.
Suzaki Y, Hamada K, Nomi T, et al. A small-molecule compound targeting CCR5 and CXCR3 prevents airway hyperresponsiveness and inflammation. Eur Respir J 2008; 31:783–789.
Yan J, Liu X, Wang Y, et al. Enhancing the potency of HBV DNA vaccines using fusion genes of HBV-specific antigens and the N-terminal fragment of gp96. J Gene Med 2007; 9:107–121.
Tan S, Gan C, Li R, et al. A novel chemosynthetic peptide with beta-sheet motif efficiently kills Klebsiella pneumoniae in a mouse model. Int J Nanomedicine 2015; 10:1045–1059.
Yang K, Wu M, Li M, et al. miR-155 suppresses bacterial clearance in Pseudomonas aeruginosa-induced keratitis by targeting Rheb. J Infect Dis 2014; 210:89–98.
Li X, He S, Zhou X, et al. Lyn delivers bacteria to lysosomes for eradication through TLR2-initiated autophagy related phagocytosis. PLoS Pathog 2016; 12:e1005363.
Luhrmann A, Haas A . A method to purify bacteria-containing phagosomes from infected macrophages. Methods Cell Sci 2000; 22:329–341.
Kannan S, Audet A, Huang H, Chen LJ, Wu M . Cholesterol-rich membrane rafts and Lyn are involved in phagocytosis during Pseudomonas aeruginosa infection. J Immunol 2008; 180:2396–2408.
Bondy-Denomy J, Garcia B, Strum S, et al. Multiple mechanisms for CRISPR-Cas inhibition by anti-CRISPR proteins. Nature 2015; 526:136–139.
Acknowledgements
We thank Dr George A O'Toole from Dartmouth Medical School for providing PA14 CRISPR-Cas components deletion and complementary mutants and vector pMQ70. We thank Dr Dominique Ferrandon of University of Strasbourg, France, for providing PA14 lasR deleting mutant and are indebted to Mary Clare Rollins and Dr Blake Wiedenheft of Montana State University for providing Csy1-4 expressing E. coli strain, indebted to Dr Jin-Town Wang from National Taiwan University for providing K. pneumonia NTUH-2044. We thank S Abrahamson (University of North Dakota Imaging Core Facility) for help with confocal imaging. This work was supported by National Institute of Health (AI109317-01A1 and AI101973-01 as well as NIH INBRE P20GM103442 and COBRE grants P20GM113123).
Author information
Authors and Affiliations
Corresponding authors
Additional information
( Supplementary information is linked to the online version of the paper on the Cell Research website.)
Supplementary information
Supplementary information, Figure S1
RNA was isolated when PA14 WT and total CRISPR-Cas cluster deletion mutant, single element deletion mutants and complementary strains grew at an OD600 nm of 1.0 in LB medium, and transcripts of indicated cas and crispr genes were quantified by qPCR. (PDF 405 kb)
Supplementary information, Figure S2
RNA was isolated when PA14 WT, ΔTCR, ΔlasR and their complementary strains grew at an OD600 nm of 2.0 in M9 medium. (PDF 171 kb)
Supplementary information, Figure S3
(A) Coomassie-blue stained SDS-polyacrylamide gel of the affinity purified Csy complexes, WT Cas3 and its mutants. (PDF 401 kb)
Supplementary information, Figure S4
Homology comparison of Cas3 amino acid sequence in PA14, E. coli UT189, PA2192, P. mendocina and P. stuzeri. (PDF 222 kb)
Supplementary information, Figure S5
Mice infected with 5 × 106 CFU/mouse with PA14 WT, ΔTCR and its complementary strain, single ΔlasR mutant and ΔTCRΔlasR double deletion mutant, respectively. (PDF 354 kb)
Supplementary information, Figure S6
Depletion of LasR alters TLR4-related host defense in mice and macrophages. (PDF 407 kb)
Supplementary information, Figure S7
Data of human AMs collaborate murine macrophage results. (PDF 399 kb)
Supplementary information, Figure S8
(A) PA14 WT, ΔTCR and its complementary strains were cultured in LB medium for 24 h shaking at 220 rpm, and then static grew in tubes for 72 h. (PDF 247 kb)
Supplementary information, Figure S9
MH-S cells were infected with K. pneumoniae NTUH-2044 and E. coli K12 respectively at an MOI of 20:1. (PDF 221 kb)
Supplementary information, Table S1
Relative normalized expression of host innate and adaptive immune response factors. (PDF 417 kb)
Supplementary information, Table S2
CRISPR-Cas and QS systems in the pathogen species1,# (PDF 598 kb)
Supplementary information, Table S3
Strains, plasmids and primers used in this study (PDF 584 kb)
Rights and permissions
About this article
Cite this article
Li, R., Fang, L., Tan, S. et al. Type I CRISPR-Cas targets endogenous genes and regulates virulence to evade mammalian host immunity. Cell Res 26, 1273–1287 (2016). https://doi.org/10.1038/cr.2016.135
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/cr.2016.135
Keywords
This article is cited by
-
CRISPR in Modulating Antibiotic Resistance of ESKAPE Pathogens
Molecular Biotechnology (2023)
-
Distribution of CRISPR-Cas systems in the Burkholderiaceae family and its biological implications
Archives of Microbiology (2022)
-
Profile of Dr. Jianxin Jiang
Science China Life Sciences (2022)
-
Engineered CRISPR-Cas systems for the detection and control of antibiotic-resistant infections
Journal of Nanobiotechnology (2021)
-
Digging into the lesser-known aspects of CRISPR biology
International Microbiology (2021)