Abstract
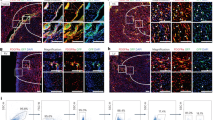
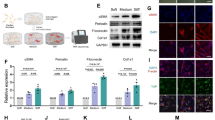
Endocardial fibroelastosis (EFE) refers to the thickening of the ventricular endocardium as a result of de novo deposition of subendocardial fibrous tissue layers during neonatal heart development. The origin of EFE fibroblasts is proposed to be postnatal endocardial cells that undergo an aberrant endothelial-to-mesenchymal transition (EndMT). Genetic lineage tracing of endocardial cells with the inducible endocardial Cre line Npr3-CreER and the endothelial cell tracing line Cdh5-CreER on an EFE-like model did not reveal any contribution of neonatal endocardial cells to fibroblasts in the EFE-like tissues. Instead, lineage tracing of embryonic epicardium by Wt1-CreER suggested that epicardium-derived mesenchymal cells (MCs) served as the major source of EFE fibroblasts. By labeling MCs using Sox9-CreER, we confirmed that MCs of the embryonic heart expand and contribute to the majority of neonatal EFE fibroblasts. During this pathological process, TGFβ signaling, the key mediator of fibroblasts activation, was highly upregulated in the EFE-like tissues. Targeting TGFβ signaling by administration of its antagonist bone morphogenetic protein 7 effectively reduced fibroblast accumulation and tissue fibrosis in the EFE-like model. Our study provides genetic evidence that excessive fibroblasts in the EFE-like tissues mainly originate from the epicardium-derived MCs through epicardial to mesenchymal transition (EpiMT). These EpiMT-derived fibroblasts within the EFE-like tissues could serve as a potential therapeutic target.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Rosahn PD . Endocardial fibroelastosis: old and new concepts. Bull NY Acad Med 1955; 31:453–472.
De Letter EA, Piette MH . Endocardial fibroelastosis as a cause of sudden unexpected death. Am J Forensic Med Pathol 1999; 20:357–363.
Stehbens WE, Delahunt B, Zuccollo JM . The histopathology of endocardial sclerosis. Cardiovasc Pathol 2000; 9:161–173.
Weinberg T, Himelfarb AJ . Endocardial fibroelastosis (so-called fetal endocarditis). A report of two cases occurring in siblings. Bull Johns Hopkins Hosp 1943; 72:299.
Grellner W, Kaferstein H, Sticht G . Combination of fatal digoxin poisoning with endocardial fibroelastosis. Forensic Sci Int 1997; 89:211–216.
Moller JH, Lucas RV Jr, Adams P Jr, Anderson RC, Jorgens J, Edwards JE . Endocardial fibroelastosis: a clinical and anatomic study of 47 patients with emphasis on its relationship to mitral insufficiency. Circulation 1964; 30:759–782.
Gilbert-Barness E, Barness LA . Nonmalformative cardiovascular pathology in infants and children. Pediatr Dev Pathol 1999; 2:499–530.
Lurie PR . Changing concepts of endocardial fibroelastosis. Cardiol Young 2010; 20:115–123.
McElhinney DB, Vogel M, Benson CB, et al. Assessment of left ventricular endocardial fibroelastosis in fetuses with aortic stenosis and evolving hypoplastic left heart syndrome. Am J Cardiol 2010; 106:1792–1797.
McElhinney DB, Marshall AC, Wilkins-Haug LE, et al. Predictors of technical success and postnatal biventricular outcome after in utero aortic valvuloplasty for aortic stenosis with evolving hypoplastic left heart syndrome. Circulation 2009; 120:1482–1490.
Emani SM, Bacha EA, McElhinney DB, et al. Primary left ventricular rehabilitation is effective in maintaining two-ventricle physiology in the borderline left heart. J Thorac Cardiovasc Surg 2009; 138:1276–1282.
Emani SM, McElhinney DB, Tworetzky W, et al. Staged left ventricular recruitment after single-ventricle palliation in patients with borderline left heart hypoplasia. J Am Coll Cardiol 2012; 60:1966–1974.
Xu X, Friehs I, Zhong Hu T, et al. Endocardial fibroelastosis is caused by aberrant endothelial to mesenchymal transition. Circ Res 2015; 116:857–866.
Merki E, Zamora M, Raya A, et al. Epicardial retinoid X receptor alpha is required for myocardial growth and coronary artery formation. Proc Natl Acad Sci USA 2005; 102:18455–18460.
Cai CL, Martin JC, Sun Y, et al. A myocardial lineage derives from Tbx18 epicardial cells. Nature 2008; 454:104–108.
Zhou B, Ma Q, Rajagopal S, et al. Epicardial progenitors contribute to the cardiomyocyte lineage in the developing heart. Nature 2008; 454:109–113.
Wilm B, Ipenberg A, Hastie ND, Burch JB, Bader DM . The serosal mesothelium is a major source of smooth muscle cells of the gut vasculature. Development 2005; 132:5317–5328.
Volz KS, Jacobs AH, Chen HI, et al. Pericytes are progenitors for coronary artery smooth muscle. eLife 2015; 4:e10036.
Moore-Morris T, Guimaraes-Camboa N, Banerjee I, et al. Resident fibroblast lineages mediate pressure overload-induced cardiac fibrosis. J Clin Invest 2014; 124:2921–2934.
Chen Q, Zhang H, Liu Y, et al. Endothelial cells are progenitors of cardiac pericytes and vascular smooth muscle cells. Nat Commun 2016; 7:12422.
Ali SR, Ranjbarvaziri S, Talkhabi M, et al. Developmental heterogeneity of cardiac fibroblasts does not predict pathological proliferation and activation. Circ Res 2014; 115:625–635.
Zeisberg EM, Tarnavski O, Zeisberg M, et al. Endothelial-to-mesenchymal transition contributes to cardiac fibrosis. Nat Med 2007; 13:952–961.
Mollmann H, Nef HM, Kostin S, et al. Bone marrow-derived cells contribute to infarct remodelling. Cardiovasc Res 2006; 71:661–671.
van Amerongen MJ, Bou-Gharios G, Popa E, et al. Bone marrow-derived myofibroblasts contribute functionally to scar formation after myocardial infarction. J Pathol 2008; 214:377–386.
Widyantoro B, Emoto N, Nakayama K, et al. Endothelial cell-derived endothelin-1 promotes cardiac fibrosis in diabetic hearts through stimulation of endothelial-to-mesenchymal transition. Circulation 2010; 121:2407–2418.
Xu J, Lin SC, Chen J, et al. CCR2 mediates the uptake of bone marrow-derived fibroblast precursors in angiotensin II-induced cardiac fibrosis. Am J Physiol Heart Circ Physiol 2011; 301:H538–547.
von Gise A, Pu WT . Endocardial and epicardial epithelial to mesenchymal transitions in heart development and disease. Circ Res 2012; 110:1628–1645.
Friehs I, Illigens B, Melnychenko I, Zhong-Hu T, Zeisberg E, Del Nido PJ . An animal model of endocardial fibroelastosis. J Surg Res 2013; 182:94–100.
Asfour B, Hare JM, Kohl T, et al. A simple new model of physiologically working heterotopic rat heart transplantation provides hemodynamic performance equivalent to that of an orthotopic heart. J Heart Lung Transplant 1999; 18:927–936.
Kong P, Christia P, Saxena A, Su Y, Frangogiannis NG . Lack of specificity of fibroblast-specific protein 1 in cardiac remodeling and fibrosis. Am J Physiol Heart Circ Physiol 2013; 305:H1363–H1372.
Zhang H, Pu W, Tian X, et al. Genetic lineage tracing identifies endocardial origin of liver vasculature. Nat Genet 2016; 48:537–543.
Zhang H, Pu W, Li G, et al. Endocardium minimally contributes to coronary endothelium in the embryonic ventricular free walls. Circ Res 2016; 118:1880–1893.
Madisen L, Zwingman TA, Sunkin SM, et al. A robust and high-throughput Cre reporting and characterization system for the whole mouse brain. Nat Neurosci 2010; 13:133–140.
Zhang H, Pu W, Liu Q, et al. Endocardium contributes to cardiac fat. Circ Res 2016; 118:254–265.
Akiyama H, Chaboissier MC, Behringer RR, et al. Essential role of Sox9 in the pathway that controls formation of cardiac valves and septa. Proc Natl Acad Sci USA 2004; 101:6502–6507.
Garside VC, Cullum R, Alder O, et al. SOX9 modulates the expression of key transcription factors required for heart valve development. Development 2015; 142:4340–4350.
Smith CL, Baek ST, Sung CY, Tallquist MD . Epicardial-derived cell epithelial-to-mesenchymal transition and fate specification require PDGF receptor signaling. Circ Res 2011; 108:e15–26.
Ranger AM, Grusby MJ, Hodge MR, et al. The transcription factor NF-ATc is essential for cardiac valve formation. Nature 1998; 392:186–190.
de la Pompa JL, Timmerman LA, Takimoto H, et al. Role of the NF-ATc transcription factor in morphogenesis of cardiac valves and septum. Nature 1998; 392:182–186.
Chang CP, Neilson JR, Bayle JH, et al. A field of myocardial-endocardial NFAT signaling underlies heart valve morphogenesis. Cell 2004; 118:649–663.
Acharya A, Baek ST, Huang G, et al. The bHLH transcription factor Tcf21 is required for lineage-specific EMT of cardiac fibroblast progenitors. Development 2012; 139:2139–2149.
Lijnen PJ, Petrov VV, Fagard RH . Induction of cardiac fibrosis by transforming growth factor-beta(1). Mol Genet Metab 2000; 71:418–435.
Bujak M, Frangogiannis NG . The role of TGF-beta signaling in myocardial infarction and cardiac remodeling. Cardiovasc Res 2007; 74:184–195.
Tomita H, Egashira K, Ohara Y, et al. Early induction of transforming growth factor-beta via angiotensin II type 1 receptors contributes to cardiac fibrosis induced by long-term blockade of nitric oxide synthesis in rats. Hypertension 1998; 32:273–279.
Nakajima H, Nakajima HO, Salcher O, et al. Atrial but not ventricular fibrosis in mice expressing a mutant transforming growth factor-beta(1) transgene in the heart. Circ Res 2000; 86:571–579.
Rosenkranz S, Flesch M, Amann K, et al. Alterations of beta-adrenergic signaling and cardiac hypertrophy in transgenic mice overexpressing TGF-beta(1). Am J Physiol Heart Circ Physiol 2002; 283:H1253–1262.
Schultz Jel J, Witt SA, Glascock BJ, et al. TGF-beta1 mediates the hypertrophic cardiomyocyte growth induced by angiotensin II. J Clin Invest 2002; 109:787–796.
Kuwahara F, Kai H, Tokuda K, et al. Transforming growth factor-beta function blocking prevents myocardial fibrosis and diastolic dysfunction in pressure-overloaded rats. Circulation 2002; 106:130–135.
Zeisberg M, Yang C, Martino M, et al. Fibroblasts derive from hepatocytes in liver fibrosis via epithelial to mesenchymal transition. J Biol Chem 2007; 282:23337–23347.
Ueha S, Shand FH, Matsushima K . Cellular and molecular mechanisms of chronic inflammation-associated organ fibrosis. Front Immunol 2012; 3:71.
Neustein HB, Lurie PR, Fugita M . Endocardial fibroelastosis found on transvascular endomyocardial biospsy in children. Arch Pathol Lab Med 1979; 103:214–219.
Kretzschmar K, Watt FM . Lineage tracing. Cell 2012; 148:33–45.
Nagy A . Cre recombinase: the universal reagent for genome tailoring. Genesis 2000; 26:99–109.
Tian X, Pu WT, Zhou B . Cellular origin and developmental program of coronary angiogenesis. Circ Res 2015; 116:515–530.
Zhang H, von Gise A, Liu Q, et al. Yap1 is required for endothelial to mesenchymal transition of the atrioventricular cushion. J Biol Chem 2014; 289:18681–18692.
Moore-Morris T, Cattaneo P, Puceat M, Evans SM . Origins of cardiac fibroblasts. J Mol Cell Cardiol 2016; 91:1–5.
Zeisberg EM, Kalluri R . Origins of cardiac fibroblasts. Circ Res 2010; 107:1304–1312.
Lane EB, Hogan BL, Kurkinen M, Garrels JI . Co-expression of vimentin and cytokeratins in parietal endoderm cells of early mouse embryo. Nature 1983; 303:701–704.
Afonso PV, McCann CP, Kapnick SM, Parent CA . Discoidin domain receptor 2 regulates neutrophil chemotaxis in 3D collagen matrices. Blood 2013; 121:1644–1650.
Hou G, Wang D, Bendeck MP . Deletion of discoidin domain receptor 2 does not affect smooth muscle cell adhesion, migration, or proliferation in response to type I collagen. Cardiovasc Pathol 2012; 21:214–218.
Kaur H, Takefuji M, Ngai CY, et al. Targeted ablation of periostin-expressing activated fibroblasts prevents adverse cardiac remodeling in mice. Circ Res 2016; 118:1906–1917.
Weber KT . Monitoring tissue repair and fibrosis from a distance. Circulation 1997; 96:2488–2492.
Chong JJ, Chandrakanthan V, Xaymardan M, et al. Adult cardiac-resident MSC-like stem cells with a proepicardial origin. Cell Stem Cell 2011; 9:527–540.
Lavine KJ, Yu K, White AC, et al. Endocardial and epicardial derived FGF signals regulate myocardial proliferation and differentiation in vivo. Dev Cell 2005; 8:85–95.
Lavine KJ, White AC, Park C, et al. Fibroblast growth factor signals regulate a wave of Hedgehog activation that is essential for coronary vascular development. Genes Dev 2006; 20:1651–1666.
Lavine KJ, Kovacs A, Ornitz DM . Hedgehog signaling is critical for maintenance of the adult coronary vasculature in mice. J Clin Invest 2008; 118:2404–2414.
de la Pompa JL, Epstein JA . Coordinating tissue interactions: Notch signaling in cardiac development and disease. Dev Cell 2012; 22:244–254.
Luna-Zurita L, Prados B, Grego-Bessa J, et al. Integration of a Notch-dependent mesenchymal gene program and Bmp2-driven cell invasiveness regulates murine cardiac valve formation. J Clin Invest 2010; 120:3493–3507.
Zhang H, Pu W, Tian X, et al. Genetic lineage tracing identifies endocardial origin of liver vasculature. Nat Genet 2016; 48:537–543.
Wang Y, Nakayama M, Pitulescu ME, et al. Ephrin-B2 controls VEGF-induced angiogenesis and lymphangiogenesis. Nature 2010; 465:483–486.
Xu Z, Wang W, Jiang K, et al. Embryonic attenuated Wnt/beta-catenin signaling defines niche location and long-term stem cell fate in hair follicle. eLife 2015; 4:e10567.
Friehs I, del Nido PJ . Increased susceptibility of hypertrophied hearts to ischemic injury. Ann Thorac Surgery 2003; 75:S678–684.
Yu W, Huang X, Tian X, et al. GATA4 regulates Fgf16 to promote heart repair after injury. Development 2016; 143:936–949.
Acknowledgements
We thank Baojin Wu, Guoyuan Chen, Zhonghui Weng and Aimin Huang for the animal husbandry; and Wei Bian for his technical help. We thank Shanghai Biomodel Organism Science & Technology Development Co., Ltd for mouse generation. We thank Ralf Adams at Max Plank Institute for providing the Cdh5-CreER mouse line and Hongkui Zeng for reporter lines. We also thank other members of our laboratory for insightful discussion and technical help throughout this study. This work was supported by Strategic Priority Research Program of the Chinese Academy of Sciences (CAS, XDB19000000), The National key Research & Development Program of China (2017YFC1001300 and 2016YFC1300600), National Natural Science Foundation of China (91639302, 31625019, 31571503, 31501172, 31601168), Youth Innovation Promotion Association of CAS (2015218), Key Project of Frontier Sciences of CAS (QYZDB-SSW-SMC003), International Cooperation Fund of CAS, National Program for Support of Top-notch Young Professionals, Shanghai Science and Technology Commission (14JC1407300, 17ZR1449600, 17ZR1449800), Shanghai Yangfan Project (15YF1414000, 16YF1413400) and Rising-Star Program (15QA1404300), China Postdoctoral Science Foundation (2015M581669, 2016T90387, 2016LH0042), President Fund of Shanghai Institutes for Biological Sciences (SIBS), Astrazeneca, Boehringer Ingelheim, Sanofi-SIBS Fellowship and Research Grants Council of Hong Kong (24110515, 14111916).
Author information
Authors and Affiliations
Corresponding authors
Additional information
( Supplementary information is linked to the online version of the paper on the Cell Research website.)
Supplementary information
Supplementary information, Figure S1
Endocardial fibroelastosis (EFE) tissue lining the left ventricle after heterotopic heart transplantation. (PDF 1240 kb)
Supplementary information, Figure S2
Npr3-CreER labeled endocardial or epicardial cells do not contribute to fibroblasts in native hearts. (PDF 1700 kb)
Supplementary information, Figure S3
Sox9 is expressed in cushion mesenchymal cells and epicardiai cells at E10.5 - E11.5. (PDF 1205 kb)
Supplementary information, Figure S4
Sox9-CreER labels embryonic cardiac mesenchymal cells and epicardial cells, but not endothelial cells. (PDF 1263 kb)
Supplementary information, Figure S5
Sox9-CreER does not label hematopoietic cells. (PDF 747 kb)
Supplementary information, Figure S6
Embryonic endocardial cells contribute to fibroblasts in the native hearts. (PDF 1181 kb)
Supplementary information, Figure S7
FSP1 is expressed in leucocytes. (PDF 1018 kb)
Rights and permissions
About this article
Cite this article
Zhang, H., Huang, X., Liu, K. et al. Fibroblasts in an endocardial fibroelastosis disease model mainly originate from mesenchymal derivatives of epicardium. Cell Res 27, 1157–1177 (2017). https://doi.org/10.1038/cr.2017.103
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/cr.2017.103
Keywords
This article is cited by
-
Tongxinluo Activates PI3K/AKT Signaling Pathway to Inhibit Endothelial Mesenchymal Transition and Attenuate Myocardial Fibrosis after Ischemia-Reperfusion in Mice
Chinese Journal of Integrative Medicine (2024)
-
Nexilin in cardiomyopathy: unveiling its diverse roles with special focus on endocardial fibroelastosis
Heart Failure Reviews (2024)
-
Endocardial fibroelastosis in infants and young children: a state-of-the-art review
Heart Failure Reviews (2023)
-
Genetic lineage tracing identifies cardiac mesenchymal-to-adipose transition in an arrhythmogenic cardiomyopathy model
Science China Life Sciences (2023)
-
Recessive ciliopathy mutations in primary endocardial fibroelastosis: a rare neonatal cardiomyopathy in a case of Alstrom syndrome
Journal of Molecular Medicine (2021)