Abstract
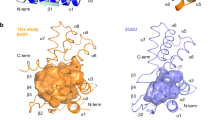
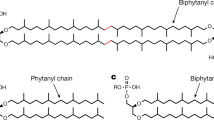
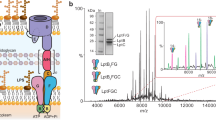
The divergence of archaea, bacteria and eukaryotes was a fundamental step in evolution. One marker of this event is a major difference in membrane lipid chemistry between these kingdoms. Whereas the membranes of bacteria and eukaryotes primarily consist of straight fatty acids ester-bonded to glycerol-3-phosphate, archaeal phospholipids consist of isoprenoid chains ether-bonded to glycerol-1-phosphate. Notably, the mechanisms underlying the biosynthesis of these lipids remain elusive. Here, we report the structure of the CDP-archaeol synthase (CarS) of Aeropyrum pernix (ApCarS) in the CTP- and Mg2+-bound state at a resolution of 2.4 Å. The enzyme comprises a transmembrane domain with five helices and cytoplasmic loops that together form a large charged cavity providing a binding site for CTP. Identification of the binding location of CTP and Mg2+ enabled modeling of the specific lipophilic substrate-binding site, which was supported by site-directed mutagenesis, substrate-binding affinity analyses, and enzyme assays. We propose that archaeol binds within two hydrophobic membrane-embedded grooves formed by the flexible transmembrane helix 5 (TM5), together with TM1 and TM4. Collectively, structural comparisons and analyses, combined with functional studies, not only elucidated the mechanism governing the biosynthesis of phospholipids with ether-bonded isoprenoid chains by CTP transferase, but also provided insights into the evolution of this enzyme superfamily from archaea to bacteria and eukaryotes.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Koga Y, Morii H . Biosynthesis of ether-type polar lipids in archaea and evolutionary considerations. Microbiol Mol Biol Rev 2007; 71:97–120.
Woese CR, Fox GE . Phylogenetic structure of the prokaryotic domain: the primary kingdoms. Proc Natl Acad Sci USA 1977; 74:5088–5090.
Jain S, Caforio A, Driessen AJ . Biosynthesis of archaeal membrane ether lipids. Front Microbiol 2014; 5:641.
Schneiter R, Toulmay A . The role of lipids in the biogenesis of integral membrane proteins. Appl Microbiol Biotechnol 2007; 73:1224–1232.
Dowhan W, Bogdanov M . Lipid-dependent membrane protein topogenesis. Annu Rev Biochem 2009; 78:515–540.
Bogdanov M, Xie J, Dowhan W . Lipid-protein interactions drive membrane protein topogenesis in accordance with the positive inside rule. J Biol Chem 2009; 284:9637–9641.
Di Paolo G, De Camilli P . Phosphoinositides in cell regulation and membrane dynamics. Nature 2006; 443:651–657.
Wymann MP, Schneiter R . Lipid signalling in disease. Nat Rev Mol Cell Biol 2008; 9:162–176.
Chaurio RA, Janko C, Munoz LE, Frey B, Herrmann M, Gaipl US . Phospholipids: key players in apoptosis and immune regulation. Molecules 2009; 14:4892–4914.
Koga Y, Morii H . Recent advances in structural research on ether lipids from archaea including comparative and physiological aspects. Biosci Biotechnol Biochem 2005; 69:2019–2034.
Koga Y . Thermal adaptation of the archaeal and bacterial lipid membranes. Archaea 2012; 2012: 789652.
Lombard J, Lopez-Garcia P, Moreira D . The early evolution of lipid membranes and the three domains of life. Nat Rev Microbiol 2012; 10:507–515.
Lombard J, Lopez-Garcia P, Moreira D . Phylogenomic investigation of phospholipid synthesis in archaea. Archaea 2012; 2012: 630910.
Icho T, Sparrow CP, Raetz CR . Molecular cloning and sequencing of the gene for CDP-diglyceride synthetase of Escherichia coli. J Biol Chem 1985; 260:12078–12083.
Sparrow CP, Raetz CR . Purification and properties of the membrane-bound CDP-diglyceride synthetase from Escherichia coli. J Biol Chem 1985; 260:12084–12091.
Langley KE, Kennedy EP . Partial purification and properties of CTP:phosphatidic acid cytidylyltransferase from membranes of Escherichia coli. J Bacteriol 1978; 136:85–95.
Kelley MJ, Carman GM . Purification and characterization of CDP-diacylglycerol synthase from Saccharomyces cerevisiae. J Biol Chem 1987; 262:14563–14570.
Shen H, Heacock PN, Clancey CJ, Dowhan W . The CDS1 gene encoding CDP-diacylglycerol synthase in Saccharomyces cerevisiae is essential for cell growth. J Biol Chem 1996; 271:789–795.
Wu L, Niemeyer B, Colley N, Socolich M, Zuker CS . Regulation of PLC-mediated signalling in vivo by CDP-diacylglycerol synthase. Nature 1995; 373:216–222.
Jain S, Caforio A, Fodran P, Lolkema JS, Minnaard AJ, Driessen AJ . Identification of CDP-archaeol synthase, a missing link of ether lipid biosynthesis in Archaea. Chem Biol 2014; 21:1392–1401.
Liu X, Yin Y, Wu J, Liu Z . Structure and mechanism of an intramembrane liponucleotide synthetase central for phospholipid biosynthesis. Nat Commun 2014; 5:4244.
Cheng W, Li W . Structural insights into ubiquinone biosynthesis in membranes. Science 2014; 343:878–881.
Huang H, Levin EJ, Liu S, Bai Y, Lockless SW, Zhou M . Structure of a membrane-embedded prenyltransferase homologous to UBIAD1. PLoS Biol 2014; 12:e1001911.
Nigou J, Besra GS . Cytidine diphosphate-diacylglycerol synthesis in Mycobacterium smegmatis. Biochem J 2002; 367:157–162.
Caffrey M . Crystallizing membrane proteins for structure determination: use of lipidic mesophases. Annu Rev Biophys 2009; 38:29–51.
Vonrhein C, Flensburg C, Keller P, et al. Data processing and analysis with the autoPROC toolbox. Acta Crystallogr D Biol Crystallogr 2011; 67:293–302.
Kabsch W . XDS. Acta Crystallogr D Biol Crystallogr 2010; 66:125–132.
Evans PR, Murshudov GN . How good are my data and what is the resolution? Acta Crystallogr D Biol Crystallogr 2013; 69:1204–1214.
Sammito M, Millan C, Frieske D, Rodriguez-Freire E, Borges RJ, Uson I . ARCIMBOLDO_LITE: single-workstation implementation and use. Acta Crystallogr D Biol Crystallogr 2015; 71:1921–1930.
Langer G, Cohen SX, Lamzin VS, Perrakis A . Automated macromolecular model building for X-ray crystallography using ARP/wARP version 7. Nat Protoc 2008; 3:1171–1179.
Adams PD, Afonine PV, Bunkoczi G, et al. PHENIX: a comprehensive Python-based system for macromolecular structure solution. Acta Crystallogr D Biol Crystallogr 2010; 66:213–221.
Emsley P, Lohkamp B, Scott WG, Cowtan K . Features and development of Coot. Acta Crystallogr D Biol Crystallogr 2010; 66:486–501.
Chen VB, Arendall WB, 3rd, Headd JJ, et al. MolProbity: all-atom structure validation for macromolecular crystallography. Acta Crystallogr D Biol Crystallogr 2010; 66:12–21.
Jones G, Willett P, Glen RC . Molecular recognition of receptor sites using a genetic algorithm with a description of desolvation. J Mol Biol 1995; 245:43–53.
Best RB, Zhu X, Shim J, et al. Optimization of the additive CHARMM all-atom protein force field targeting improved sampling of the backbone phi, psi and side-chain chi(1) and chi(2) dihedral angles. J Chem Theory Comput 2012; 8:3257–3273.
Vanommeslaeghe K, MacKerell AD Jr. Automation of the CHARMM General Force Field (CGenFF) I: bond perception and atom typing. J Chem Inf Model 2012; 52:3144–3154.
Lee J, Cheng X, Swails JM, et al. CHARMM-GUI input generator for NAMD, GROMACS, AMBER, OpenMM, and CHARMM/OpenMM simulations using the CHARMM36 additive force field. J Chem Theory Comput 2016; 12:405–413.
Pronk S, Pall S, Schulz R, et al. GROMACS 4.5: a high-throughput and highly parallel open source molecular simulation toolkit. Bioinformatics 2013; 29:845–854.
Dowler S, Kular G, Alessi DR . Protein lipid overlay assay. Sci STKE 2002; 2002:pl6.
Robert X, Gouet P . Deciphering key features in protein structures with the new ENDscript server. Nucleic Acids Res 2014; 42:W320–324.
Acknowledgements
We thank the staff of the BL19U1 beamline at the National Center for Protein Sciences Shanghai (NCPSS) at the Shanghai Synchrotron Radiation Facility for assistance with data collection. We thank J Chai for critical comments. We thank Peter Fodran and Adriaan Minnaard for the synthesis of DGGGP. This work was funded by the National Natural Science Foundation of China (NSFC; 31570842), the National Young Thousand Talents Program of China, and the Sichuan Province Thousand Talents Program to WC. AC was supported by a research program from the biobased ecologically balanced sustainable industrial chemistry (BE-BASIC). Computational support was provided by the special Program for Applied Research on Super Computation of the NSFC-Guangdong Joint Fund (the second phase; U1501501).
Author information
Authors and Affiliations
Corresponding authors
Additional information
( Supplementary information is linked to the online version of the paper on the Cell Research website.)
Supplementary information
Supplementary information, Figure S1
Phylogenetic tree distribution of members of the archaeal and bacterial CTP transferases families. (PDF 423 kb)
Supplementary information, Figure S2
Amino acid sequence alignment of CarS homologs in three life kingdoms. (PDF 1042 kb)
Supplementary information, Figure S3
Detection of products catalyzed by ApCarS using high performance liquid chromatography-mass spectrometry (HPLC-MS). (PDF 195 kb)
Supplementary information, Figure S4
Activity assays of ApCarS in the presence of different ions. (PDF 281 kb)
Supplementary information, Figure S5
Stabilization of cytoplasmic loop 1 (CL1) is essential for the CTP-binding and catalytic activity of ApCarS mutants. (PDF 396 kb)
Supplementary information, Figure S6
Electron density maps for the structure of ApCarS bound to CTP and metal ions. (PDF 564 kb)
Supplementary information, Figure S7
The stabilization of the periplasmic region is required for the enzymatic activity of ApCarS. (PDF 302 kb)
Supplementary information, Figure S8
Isothermal titration calorimetry (ITC) measurements of the CTP-binding activity of ApCarS encoding amino acid substitutions in the cytoplasmic domain (CPD). (PDF 848 kb)
Supplementary information, Figure S9
Structural basis for this catalytic mechanism. (PDF 900 kb)
Supplementary information, Figure S10
Structural analysis the potential binding site of Archaeol. (PDF 208 kb)
Supplementary information, Table S1
Data collect and refinement statistics (PDF 116 kb)
Supplementary information, Table S2
Representative affinity constants and binding parameters demonstrating the effect of amino acid substitutions within the cytoplasmic domain (CPD) of ApCarS on CTP-binding (PDF 70 kb)
Rights and permissions
About this article
Cite this article
Ren, S., Caforio, A., Yang, Q. et al. Structural and mechanistic insights into the biosynthesis of CDP-archaeol in membranes. Cell Res 27, 1378–1391 (2017). https://doi.org/10.1038/cr.2017.122
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/cr.2017.122
Keywords
This article is cited by
-
The catalytic and structural basis of archaeal glycerophospholipid biosynthesis
Extremophiles (2022)