Abstract
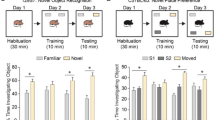
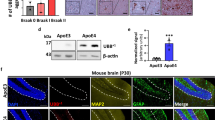
The autism spectrum disorders (ASDs) are a collection of human neurological disorders with heterogeneous etiologies. Hyperactivity of E3 ubiquitin (Ub) ligase UBE3A, stemming from 15q11-q13 copy number variations, accounts for 1%-3% of ASD cases worldwide, but the underlying mechanisms remain incompletely characterized. Here we report that the functionality of ALDH1A2, the rate-limiting enzyme of retinoic acid (RA) synthesis, is negatively regulated by UBE3A in a ubiquitylation-dependent manner. Excessive UBE3A dosage was found to impair RA-mediated neuronal homeostatic synaptic plasticity. ASD-like symptoms were recapitulated in mice by overexpressing UBE3A in the prefrontal cortex or by administration of an ALDH1A antagonist, whereas RA supplements significantly alleviated excessive UBE3A dosage-induced ASD-like phenotypes. By identifying reduced RA signaling as an underlying mechanism in ASD phenotypes linked to UBE3A hyperactivities, our findings introduce a new vista of ASD etiology and facilitate a mode of therapeutic development against this increasingly prevalent disease.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Huguet G, Ey E, Bourgeron T . The genetic landscapes of autism spectrum disorders. Annu Rev Genomics Hum Genet 2013; 14:191–213.
Zoghbi HY, Bear MF . Synaptic dysfunction in neurodevelopmental disorders associated with autism and intellectual disabilities. Cold Spring Harb Perspect Biol 2012; 4.
Abrahams BS, Geschwind DH . Advances in autism genetics: on the threshold of a new neurobiology. Nat Rev Genet 2008; 9:341–355.
Glessner JT, Wang K, Cai G, et al. Autism genome-wide copy number variation reveals ubiquitin and neuronal genes. Nature 2009; 459:569–573.
De Rubeis S, He X, Goldberg AP, et al. Synaptic, transcriptional and chromatin genes disrupted in autism. Nature 2014; 515:209–215.
Iossifov I, O'Roak BJ, Sanders SJ, et al. The contribution of de novo coding mutations to autism spectrum disorder. Nature 2014; 515:216–221.
Sanders SJ, He X, Willsey AJ, et al. Insights into autism spectrum disorder genomic architecture and biology from 71 risk loci. Neuron 2015; 87:1215–1233.
Wang T, Guo H, Xiong B, et al. De novo genic mutations among a Chinese autism spectrum disorder cohort. Nat Commun 2016; 7:13316.
Doan RN, Bae BI, Cubelos B, et al. Mutations in human accelerated regions disrupt cognition and social behavior. Cell 2016; 167:341–354.
Toro R, Konyukh M, Delorme R, et al. Key role for gene dosage and synaptic homeostasis in autism spectrum disorders. Trends Genet 2010; 26:363–372.
Santini E, Klann E . Reciprocal signaling between translational control pathways and synaptic proteins in autism spectrum disorders. Sci Signal 2014; 7:re10.
Schanen NC . Epigenetics of autism spectrum disorders. Hum Mol Genet 2006; 15:R138–R150.
Nurmi EL, Bradford Y, Chen Y, et al. Linkage disequilibrium at the Angelman syndrome gene UBE3A in autism families. Genomics 2001; 77:105–113.
Baron CA . Genomic and functional profiling of duplicated chromosome 15 cell lines reveal regulatory alterations in UBE3A-associated ubiquitin-proteasome pathway processes. Hum Mol Genet 2006; 15:853–869.
Noor A, Dupuis L, Mittal K, et al. 15q11.2 Duplication encompassing only the UBE3A gene is associated with developmental delay and neuropsychiatric phenotypes. Hum Mutat 2015; 36:689–693.
Smith SE, Zhou YD, Zhang G, et al. Increased gene dosage of Ube3a results in autism traits and decreased glutamate synaptic transmission in mice. Sci Transl Med 2011; 3:103ra197.
Yi JJ, Berrios J, Newbern JM, et al. An autism-linked mutation disables phosphorylation control of UBE3A. Cell 2015; 162:795–807.
Grabbe C, Husnjak K, Dikic I . The spatial and temporal organization of ubiquitin networks. Nat Rev Mol Cell Biol 2011; 12:295–307.
Hershko A, Ciechanover A, Varshavsky A . Basic Medical Research Award. The ubiquitin system. Nat Med 2000; 6:1073–1081.
Kerscher O, Felberbaum R, Hochstrasser M . Modification of proteins by ubiquitin and ubiquitin-like proteins. Annu Rev Cell Dev Biol 2006; 22:159–180.
Komander D, Rape M . The ubiquitin code. Annu Rev Biochem 2012; 81:203–229.
Swatek KN, Komander D . Ubiquitin modifications. Cell Res 2016; 26:399–422.
Sell GL, Margolis SS . From UBE3A to Angelman syndrome: a substrate perspective. Front Neurosci 2015; 9:322.
Kumar S, Duester G . SnapShot: retinoic acid signaling. Cell 2011; 147:1422.
Shearer KD, Stoney PN, Morgan PJ, McCaffery PJ . A vitamin for the brain. Trends Neurosci 2012; 35:733–741.
Rhinn M, Dolle P . Retinoic acid signalling during development. Development 2012; 139:843–858.
Cunningham TJ, Duester G . Mechanisms of retinoic acid signalling and its roles in organ and limb development. Nat Rev Mol Cell Biol 2015; 16:110–123.
Chen L, Lau AG, Sarti F . Synaptic retinoic acid signaling and homeostatic synaptic plasticity. Neuropharmacology 2014; 78:3–12.
Aoto J, Nam CI, Poon MM, Ting P, Chen L . Synaptic signaling by all-trans retinoic acid in homeostatic synaptic plasticity. Neuron 2008; 60:308–320.
Chen N, Napoli JL . All-trans-retinoic acid stimulates translation and induces spine formation in hippocampal neurons through a membrane-associated RARα. FASEB J 2008; 22:236–245.
Koppaka V, Thompson DC, Chen Y, et al. Aldehyde dehydrogenase inhibitors: a comprehensive review of the pharmacology, mechanism of action, substrate specificity, and clinical application. Pharmacol Rev 2012; 64:520–539.
Keren-Kaplan T, Attali I, Motamedchaboki K, et al. Synthetic biology approach to reconstituting the ubiquitylation cascade in bacteria. EMBO J 2012; 31:378–390.
Moreb JS, Zucali JR, Ostmark B, Benson NA . Heterogeneity of aldehyde dehydrogenase expression in lung cancer cell lines is revealed by Aldefluor flow cytometry-based assay. Cytometry B Clin Cytom 2007; 72:281–289.
Jiang YH, Armstrong D, Albrecht U, et al. Mutation of the Angelman ubiquitin ligase in mice causes increased cytoplasmic p53 and deficits of contextual learning and long-term potentiation. Neuron 1998; 21:799–811.
Yamamoto Y, Huibregtse JM, Howley PM . The human E6-AP gene (UBE3A) encodes three potential protein isoforms generated by differential splicing. Genomics 1997; 41:263–266.
Kumar S, Talis AL, Howley PM . Identification of HHR23A as a substrate for E6-associated protein-mediated ubiquitination. J Biol Chem 1999; 274:18785–18792.
Louria-Hayon I, Alsheich-Bartok O, Levav-Cohen Y, et al. E6AP promotes the degradation of the PML tumor suppressor. Cell Death Differ 2009; 16:1156–1166.
Zaaroor-Regev D, de Bie P, Scheffner M, et al. Regulation of the polycomb protein Ring1B by self-ubiquitination or by E6-AP may have implications to the pathogenesis of Angelman syndrome. Proc Natl Acad Sci USA 2010; 107:6788–6793.
Li W, Yao A, Zhi H, et al. Angelman syndrome protein Ube3a regulates synaptic growth and endocytosis by inhibiting BMP signaling in Drosophila. PLoS Genet 2016; 12:e1006062.
Huibregtse JM, Scheffner M, Howley PM . Localization of the E6-AP regions that direct human papillomavirus E6 binding, association with p53, and ubiquitination of associated proteins. Mol Cell Biol 1993; 13:4918–4927.
Ansari T, Brimer N, Vande Pol SB . Peptide interactions stabilize and restructure human papillomavirus type 16 E6 to interact with p53. J Virol 2012; 86:11386–11391.
Moretti A, Li J, Donini S, et al. Crystal structure of human aldehyde dehydrogenase 1A3 complexed with NAD+ and retinoic acid. Sci Rep 2016; 6:35710.
Storms RW, Trujillo AP, Springer JB, et al. Isolation of primitive human hematopoietic progenitors on the basis of aldehyde dehydrogenase activity. Proc Natl Acad Sci USA 1999; 96:9118–9123.
Han EB, Stevens CF . Development regulates a switch between post- and presynaptic strengthening in response to activity deprivation. Proc Natl Acad Sci USA 2009; 106:10817–10822.
Bicks LK, Koike H, Akbarian S, Morishita H . Prefrontal cortex and social cognition in mouse and man. Front Psychol 2015; 6:1805.
Barak B, Feng G . Neurobiology of social behavior abnormalities in autism and Williams syndrome. Nat Neurosci 2016; 19:647–655.
Chow ML, Pramparo T, Winn ME, et al. Age-dependent brain gene expression and copy number anomalies in autism suggest distinct pathological processes at young versus mature ages. PLoS Genet 2012; 8:e1002592.
Stoner R, Chow ML, Boyle MP, et al. Patches of disorganization in the neocortex of children with autism. N Engl J Med 2014; 370:1209–1219.
Duffney LJ, Zhong P, Wei J, et al. Autism-like deficits in Shank3-deficient mice are rescued by targeting actin regulators. Cell Rep 2015; 11:1400–1413.
Lein ES, Hawrylycz MJ, Ao N, et al. Genome-wide atlas of gene expression in the adult mouse brain. Nature 2007; 445:168–176.
Kane MA, Napoli JL . Quantification of endogenous retinoids. Methods Mol Biol 2010; 652:1–54.
Evans JE, McCaffery P . HPLC/MS(N) analysis of retinoids. Methods Mol Biol 2010; 652:149–162.
Ramocki MB, Peters SU, Tavyev YJ, et al. Autism and other neuropsychiatric symptoms are prevalent in individuals with MeCP2 duplication syndrome. Ann Neurol 2009; 66:771–782.
Durand CM, Betancur C, Boeckers TM, et al. Mutations in the gene encoding the synaptic scaffolding protein SHANK3 are associated with autism spectrum disorders. Nat Genet 2007; 39:25–27.
Zweier C, de Jong EK, Zweier M, et al. CNTNAP2 and NRXN1 are mutated in autosomal-recessive Pitt-Hopkins-like mental retardation and determine the level of a common synaptic protein in Drosophila. Am J Hum Genet 2009; 85:655–666.
Ramocki MB, Zoghbi HY . Failure of neuronal homeostasis results in common neuropsychiatric phenotypes. Nature 2008; 455:912–918.
Misner DL, Jacobs S, Shimizu Y, et al. Vitamin A deprivation results in reversible loss of hippocampal long-term synaptic plasticity. Proc Natl Acad Sci USA 2001; 98:11714–11719.
Sarti F, Schroeder J, Aoto J, Chen L . Conditional RARα knockout mice reveal acute requirement for retinoic acid and RARα in homeostatic plasticity. Front Mol Neurosci 2012; 5:16.
Wan C, Shi Y, Zhao X, et al. Positive association between ALDH1A2 and schizophrenia in the Chinese population. Prog Neuropsychopharmacol Biol Psychiatry 2009; 33:1491–1495.
Galter D, Buervenich S, Carmine A, Anvret M, Olson L . ALDH1 mRNA: presence in human dopamine neurons and decreases in substantia nigra in Parkinson's disease and in the ventral tegmental area in schizophrenia. Neurobiol Dis 2003; 14:637–647.
Fares-Taie L, Gerber S, Chassaing N, et al. ALDH1A3 mutations cause recessive anophthalmia and microphthalmia. Am J Hum Genet 2013; 92:265–270.
Ramamoorthy S, Nawaz Z . E6-associated protein (E6-AP) is a dual function coactivator of steroid hormone receptors. Nucl Recept Signal 2008; 6:e006.
Krishnan V, Stoppel DC, Nong Y, et al. Autism gene Ube3a and seizures impair sociability by repressing VTA Cbln1. Nature 2017; 543:507–512.
Sztainberg Y, Zoghbi HY . Lessons learned from studying syndromic autism spectrum disorders. Nat Neurosci 2016; 19:1408–1417.
Delorme R, Ey E, Toro R, et al. Progress toward treatments for synaptic defects in autism. Nat Med 2013; 19:685–694.
Xia K, Guo H, Hu Z, et al. Common genetic variants on 1p13.2 associate with risk of autism. Mol Psychiatry 2013; 19:1212–1219.
Nava C, Keren B, Mignot C, et al. Prospective diagnostic analysis of copy number variants using SNP microarrays in individuals with autism spectrum disorders. Eur J Hum Genet 2013; 22:71–78.
Benn P, Delach J . Human lymphocyte culture and chromosome analysis. CSH Protoc 2008; 2008:pdbprot5035.
Xu X, Tao Y, Gao X, et al. A CRISPR-based approach for targeted DNA demethylation. Cell Discov 2016; 2:16009.
Anderson MA, Gusella JF . Use of cyclosporin A in establishing Epstein-Barr virus-transformed human lymphoblastoid cell lines. In Vitro 1984; 20:856–858.
Xu J . Preparation, culture, and immortalization of mouse embryonic fibroblasts. Curr Protoc Mol Biol 2005; Chapter 28:Unit 28.1.
Hsu PD, Scott DA, Weinstein JA, et al. DNA targeting specificity of RNA-guided Cas9 nucleases. Nat Biotechnol 2013; 31:827–832.
Liu H, Naismith JH . An efficient one-step site-directed deletion, insertion, single and multiple-site plasmid mutagenesis protocol. BMC Biotechnol 2008; 8:91.
Liu Z, Chen P, Gao H, et al. Ubiquitylation of autophagy receptor Optineurin by HACE1 activates selective autophagy for tumor suppression. Cancer Cell 2014; 26:106–120.
Sztainberg Y, Chen H-m, Swann JW, et al. Reversal of phenotypes in MECP2 duplication mice using genetic rescue or antisense oligonucleotides. Nature 2015; 528:123–126.
Acknowledgements
We thank Prof Qishui Lin (SIBCB, CAS) for invaluable advice. We appreciate Drs Xiang Yu, Jiulin Du, Bo Yuan (Institute of Neuroscience, SIBS, CAS), Donghong Cui, Han Li (Shanghai Mental Health Center) for providing technical assistance, and Zi Li (Institute of Nutritional Sciences, SIBS, CAS) for technical help in HPLC-MS/MS. We specially acknowledge the excellent support from proteomics facility at the National Center for Protein Science Shanghai, and the cell imaging center led by Dr Wei Bian at SIBCB. We thank all members of our laboratory for support and Ms Yalan Wu for assistance. We are also grateful to Dr ZeNan Chang (University of California, Los Angeles) for critical reading of the manuscript. This work was supported by the Strategic Priority Research Program of the Chinese Academy of Sciences (XDB19000000 and XDA12040323), the National Natural Science Foundation of China (31470770 and 81525019 to RH; 81601203 to XX; 81330027 and 81525007 to KX; 31400919 and 31671114 to HG), the Ministry of Science and Technology of China (2013CB910900 to RH) and the China Postdoctoral Science Foundation (2016M591724 to XX).
Author information
Authors and Affiliations
Corresponding author
Additional information
( Supplementary information is linked to the online version of the paper on the Cell Research website.)
Supplementary information
Supplementary information, Figure S1
UBE3A interacts with, and ubiquitylates other members of human ALDH1A family. (PDF 462 kb)
Supplementary information, Figure S2
The members of mammalian ALDH1A family proteins are highly conserved in both amino acid sequences and secondary structures. (PDF 169 kb)
Supplementary information, Figure S3
Low abundances of endogenous ALDH1A family proteins in H1299 cell line and its derivative H1299 UBE3A−/− cells. (PDF 163 kb)
Supplementary information, Figure S4
UBE3A ubiquitylates endogenous or exogenous ALDH1A2 protein in a dosage-dependent manner. (PDF 641 kb)
Supplementary information, Figure S5
Copy number amplification identified in three autism patients. (PDF 379 kb)
Supplementary information, Figure S6
Ubiquitylation of ALDH1A2 compromises its dehydrogenase activity, using propionaldehyde as substrate. (PDF 202 kb)
Supplementary information, Figure S7
Neuronal activity blockade induced changes in synaptic homeostasis through translational control. (PDF 175 kb)
Supplementary information, Figure S8
Stereotaxic injection of indicated AAVs into mouse PFC regions. (PDF 461 kb)
Supplementary information, Figure S8
Mouse behavior tests for mice after stereotaxic injection of indicated AAVs or those receiving oral intake of ATRA. (PDF 137 kb)
Supplementary information, Figure S10
Administration with disulfiram did not alter the mouse behaviors in open-field, elevated plus maze or rotarod tests. (PDF 114 kb)
Supplementary information, Table S1
Methylation analysis of SNRPN gene in ASD patients with 15q11-13 duplication. (PDF 152 kb)
Supplementary information, Table S2
Plasmids used in this study. (PDF 90 kb)
Supplementary information, Table S3
qRT-PCR primers used in this study. (PDF 125 kb)
Rights and permissions
About this article
Cite this article
Xu, X., Li, C., Gao, X. et al. Excessive UBE3A dosage impairs retinoic acid signaling and synaptic plasticity in autism spectrum disorders. Cell Res 28, 48–68 (2018). https://doi.org/10.1038/cr.2017.132
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/cr.2017.132
Keywords
This article is cited by
-
The lifespan continuum of brain disorders: investigating links between neurodevelopmental and neurodegenerative disease—chicken or egg?
Journal of Neural Transmission (2026)
-
Retinoic acid-mediated homeostatic plasticity drives cell type-specific CP-AMPAR accumulation in nucleus accumbens core and incubation of cocaine craving
Molecular Psychiatry (2025)
-
Ubiquitination in the Nervous System: From Molecular Mechanisms to Disease Implications
Molecular Neurobiology (2025)
-
NEAT1 Promotes Valproic Acid-Induced Autism Spectrum Disorder by Recruiting YY1 to Regulate UBE3A Transcription
Molecular Neurobiology (2025)
-
Prenatal Exposure to Azadiradione Leads to Developmental Disabilities
Molecular Neurobiology (2025)