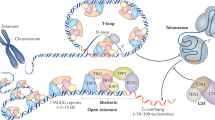
Abstract
Telomeres are nucleoprotein complexes that play essential roles in protecting chromosome ends. Mammalian telomeres consist of repetitive DNA sequences bound by the shelterin complex. In this complex, the POT1-TPP1 heterodimer binds to single-stranded telomeric DNAs, while TRF1 and TRF2-RAP1 interact with double-stranded telomeric DNAs. TIN2, the linchpin of this complex, simultaneously interacts with TRF1, TRF2, and TPP1 to mediate the stable assembly of the shelterin complex. However, the molecular mechanism by which TIN2 interacts with these proteins to orchestrate telomere protection remains poorly understood. Here, we report the crystal structure of the N-terminal domain of TIN2 in complex with TIN2-binding motifs from TPP1 and TRF2, revealing how TIN2 interacts cooperatively with TPP1 and TRF2. Unexpectedly, TIN2 contains a telomeric repeat factor homology (TRFH)-like domain that functions as a protein-protein interaction platform. Structure-based mutagenesis analyses suggest that TIN2 plays an important role in maintaining the stable shelterin complex required for proper telomere end protection.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
Accession codes
References
Palm W, de Lange T . How shelterin protects mammalian telomeres. Annu Rev Genet 2008; 42:301–334.
de Lange T . Shelterin: the protein complex that shapes and safeguards human telomeres. Genes Dev 2005; 19:2100–2110.
de Lange T . How telomeres solve the end-protection problem. Science 2009; 326:948–952.
Chen Y, Yang Y, van Overbeek M, et al. A shared docking motif in TRF1 and TRF2 used for differential recruitment of telomeric proteins. Science 2008; 319:1092–1096.
Kim H, Lee OH, Xin H, et al. TRF2 functions as a protein hub and regulates telomere maintenance by recognizing specific peptide motifs. Nat Struct Mol Biol 2009; 16:372–379.
Rai R, Hu C, Broton C, Chen Y, Lei M, Chang S . NBS1 phosphorylation status dictates repair choice of dysfunctional telomeres. Mol Cell 2017; 65:801–817.e4.
Wang F, Podell ER, Zaug AJ, et al. The POT1-TPP1 telomere complex is a telomerase processivity factor. Nature 2007; 445:506–510.
Xin H, Liu D, Wan M, et al. TPP1 is a homologue of ciliate TEBP-beta and interacts with POT1 to recruit telomerase. Nature 2007; 445:559–562.
Nandakumar J, Bell CF, Weidenfeld I, Zaug AJ, Leinwand LA, Cech TR . The TEL patch of telomere protein TPP1 mediates telomerase recruitment and processivity. Nature 2012; 492:285–289.
Denchi EL, de Lange T . Protection of telomeres through independent control of ATM and ATR by TRF2 and POT1. Nature 2007; 448:1068–1071.
Guo X, Deng Y, Lin Y, et al. Dysfunctional telomeres activate an ATM-ATR-dependent DNA damage response to suppress tumorigenesis. EMBO J 2007; 26:4709–4719.
Wu L, Multani AS, He H, et al. Pot1 deficiency initiates DNA damage checkpoint activation and aberrant homologous recombination at telomeres. Cell 2006; 126:49–62.
He H, Multani AS, Cosme-Blanco W, et al. POT1b protects telomeres from end-to-end chromosomal fusions and aberrant homologous recombination. EMBO J 2006; 25:5180–5190.
Hockemeyer D, Daniels JP, Takai H, de Lange T . Recent expansion of the telomeric complex in rodents: two distinct POT1 proteins protect mouse telomeres. Cell 2006; 126:63–77.
Kibe T, Zimmermann M, de Lange T . TPP1 blocks an ATR-mediated resection mechanism at telomeres. Mol Cell 2016; 61:236–246.
Kim SH, Kaminker P, Campisi J . TIN2, a new regulator of telomere length in human cells. Nat Genet 1999; 23:405–412.
Ye JZ, Donigian JR, van Overbeek M, et al. TIN2 binds TRF1 and TRF2 simultaneously and stabilizes the TRF2 complex on telomeres. J Biol Chem 2004; 279:47264–47271.
Frescas D, de Lange T . Binding of TPP1 protein to TIN2 protein is required for POT1a,b protein-mediated telomere protection. J Biol Chem 2014; 289:24180–24187.
Frescas D, de Lange T . TRF2-tethered TIN2 can mediate telomere protection by TPP1/POT1. Mol Cell Biol 2014; 34:1349–1362.
Kim SH, Beausejour C, Davalos AR, Kaminker P, Heo SJ, Campisi J . TIN2 mediates functions of TRF2 at human telomeres. J Biol Chem 2004; 279:43799–43804.
O'Connor MS, Safari A, Xin H, Liu D, Songyang Z . A critical role for TPP1 and TIN2 interaction in high-order telomeric complex assembly. Proc Natl Acad Sci USA 2006; 103:11874–11879.
Takai KK, Kibe T, Donigian JR, Frescas D, de Lange T . Telomere protection by TPP1/POT1 requires tethering to TIN2. Mol Cell 2011; 44:647–659.
Frank AK, Tran DC, Qu RW, Stohr BA, Segal DJ, Xu L . The Shelterin TIN2 subunit mediates recruitment of telomerase to telomeres. PLoS Genet 2015; 11:e1005410.
Ye JZ, de Lange T . TIN2 is a tankyrase 1 PARP modulator in the TRF1 telomere length control complex. Nat Genet 2004; 36:618–623.
Zeng Z, Wang W, Yang Y, et al. Structural basis of selective ubiquitination of TRF1 by SCFFbx4. Dev Cell 2010; 18:214–225.
Liu D, Safari A, O'Connor MS, et al. PTOP interacts with POT1 and regulates its localization to telomeres. Nat Cell Biol 2004; 6:673–680.
Holm L, Rosenstrom P . Dali server: conservation mapping in 3D. Nucleic Acids Res 2010; 38:W545–W549.
Imielinski M, Berger AH, Hammerman PS, et al. Mapping the hallmarks of lung adenocarcinoma with massively parallel sequencing. Cell 2012; 150:1107–1120.
Boora GK, Kanwar R, Kulkarni AA, et al. Exome-level comparison of primary well-differentiated neuroendocrine tumors and their cell lines. Cancer Genet 2015; 208:374–381.
Attwooll CL, Akpinar M, Petrini JH . The mre11 complex and the response to dysfunctional telomeres. Mol Cell Biol 2009; 29:5540–5551.
Deng Y, Guo X, Ferguson DO, Chang S . Multiple roles for MRE11 at uncapped telomeres. Nature 2009; 460:914–918.
Dimitrova N, de Lange T . Cell cycle-dependent role of MRN at dysfunctional telomeres: ATM signaling-dependent induction of nonhomologous end joining (NHEJ) in G1 and resection-mediated inhibition of NHEJ in G2. Mol Cell Biol 2009; 29:5552–5563.
Rai R, Zheng H, He H, et al. The function of classical and alternative non-homologous end-joining pathways in the fusion of dysfunctional telomeres. EMBO J 2010; 29:2598–2610.
Wang M, Wu W, Rosidi B, Zhang L, Wang H, Iliakis G . PARP-1 and Ku compete for repair of DNA double strand breaks by distinct NHEJ pathways. Nucleic Acids Res 2006; 34:6170–6182.
Yan CT, Boboila C, Souza EK, et al. IgH class switching and translocations use a robust non-classical end-joining pathway. Nature 2007; 449:478–482.
Boboila C, Yan C, Wesemann DR, et al. Alternative end-joining catalyzes class switch recombination in the absence of both Ku70 and DNA ligase 4. J Exp Med 2010; 207:417–427.
Simsek D, Jasin M . Alternative end-joining is suppressed by the canonical NHEJ component Xrcc4-ligase IV during chromosomal translocation formation. Nat Struct Mol Biol 2010; 17:410–416.
Sfeir A, de Lange T . Removal of shelterin reveals the telomere end-protection problem. Science 2012; 336:593–597.
Chen Y, Rai R, Zhou ZR, et al. A conserved motif within RAP1 has diversified roles in telomere protection and regulation in different organisms. Nat Struct Mol Biol 2011; 18:213–221.
Chen C, Gu P, Wu J, et al. Structural insights into POT1-TPP1 interaction and POT1 C-terminal mutations in human cancer. Nat Commun 2017; 8:14929.
Rice C, Shastrula PK, Kossenkov AV, et al. Structural and functional analysis of the human POT1-TPP1 telomeric complex. Nat Commun 2017; 8:14928.
Shi T, Bunker RD, Mattarocci S, et al. Rif1 and Rif2 shape telomere function and architecture through multivalent Rap1 interactions. Cell 2013; 153:1340–1353.
Spengler D . The protein kinase Aurora C phosphorylates TRF2. Cell Cycle 2007; 6:2579–2580.
Fairall L, Chapman L, Moss H, de Lange T, Rhodes D . Structure of the TRFH dimerization domain of the human telomeric proteins TRF1 and TRF2. Mol Cell 2001; 8:351–361.
Broccoli D, Smogorzewska A, Chong L, de Lange T . Human telomeres contain two distinct Myb-related proteins, TRF1 and TRF2. Nat Genet 1997; 17:231–235.
Benarroch-Popivker D, Pisano S, Mendez-Bermudez A, et al. TRF2-mediated control of telomere DNA topology as a mechanism for chromosome-end protection. Mol Cell 2016; 61:274–286.
Chen LY, Zhang Y, Zhang Q, et al. Mitochondrial localization of telomeric protein TIN2 links telomere regulation to metabolic control. Mol Cell 2012; 47:839–850.
Lam YC, Akhter S, Gu P, et al. SNMIB/Apollo protects leading-strand telomeres against NHEJ-mediated repair. EMBO J 2010; 29:2230–2241.
Otwinowski Z, Minor W . Processing of X-ray Diffraction Data Collected in Oscillation Mode Methods in Enzymology. New York: Academic Press 1997:307–326.
Vonrhein C, Blanc E, Roversi P, Bricogne G . Automated structure solution with autoSHARP. Methods Mol Biol 2007; 364:215–230.
Adams PD, Grosse-Kunstleve RW, Hung LW, et al. PHENIX: building new software for automated crystallographic structure determination. Acta Crystallogr D Biol Crystallogr 2002; 58:1948–1954.
Emsley P, Cowtan K . Coot: model-building tools for molecular graphics. Acta Crystallogr D Biol Crystallogr 2004; 60:2126–2132.
Takai H, Smogorzewska A, de Lange T . DNA damage foci at dysfunctional telomeres. Curr Biol 2003; 13:1549–1556.
Rai R, Chang S . Probing the telomere damage response. Methods Mol Biol 2017; 1587:133–138.
Acknowledgements
We thank staffs from BL18U1 and BL19U1 beamlines at NCPSS and Shanghai Synchrotron Radiation Facility (SSRF) for their help with crystal data collection. We are extremely grateful to National Center for Protein Sciences Shanghai (Protein Expression and Purification system, NMR system) for their instrument support and technical assistance. This work was supported by grants from the Strategic Priority Research Program of the Chinese Academy of Sciences (XDB08010201) to ML and YC, the Ministry of Science and Technology of China (2013CB910401 to YC,2013CB910402 to ML), the National Natural Science Foundation of China (31470737 and 31670748 to YC, 31330040 and 31525007 to ML), the Basic Research Project of Shanghai Science and Technology Commission (14JC1407200 to YC), and NCI (RO1 CA129037, RO1CA202816, R21CA200506, and R21CA182280) to SC.
Author information
Authors and Affiliations
Corresponding authors
Additional information
( Supplementary information is linked to the online version of the paper on the Cell Research website.)
Supplementary information
Supplementary information, Figure S1
Mapping interaction domains between TRF2-TIN2 and TPP1-TIN2. (PDF 443 kb)
Supplementary information, Figure S2
The crystal structure of TIN2TRFH-TPP1TBM-TRF2TBM complex. (PDF 2180 kb)
Supplementary information, Figure S3
Analyses of TIN2 localization in U2OS cells. (PDF 140 kb)
Supplementary information, Figure S4
TIN2 protects telomeres from engaging in DNA damage responses. (PDF 205 kb)
Supplementary information, Figure S5
TIN2 interaction with shelterin components is required to prevent chromosome fusions. (PDF 2125 kb)
Supplementary information, Figure S6
TIN2TRFH shows distinct properties compared with TRF2TRFH. (PDF 854 kb)
Supplementary information, Figure S7
TIN2132-188 is crucial for both TRF2 and TPP1 interactions, and also maintains the correct fold of TIN2TRFH. (PDF 153 kb)
Rights and permissions
About this article
Cite this article
Hu, C., Rai, R., Huang, C. et al. Structural and functional analyses of the mammalian TIN2-TPP1-TRF2 telomeric complex. Cell Res 27, 1485–1502 (2017). https://doi.org/10.1038/cr.2017.144
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/cr.2017.144
Keywords
This article is cited by
-
TRF1 and TRF2: pioneering targets in telomere-based cancer therapy
Journal of Cancer Research and Clinical Oncology (2024)
-
Potential effects of assisted reproductive technology on telomere length and telomerase activity in human oocytes and early embryos
Journal of Ovarian Research (2023)
-
Shaping human telomeres: from shelterin and CST complexes to telomeric chromatin organization
Nature Reviews Molecular Cell Biology (2021)
-
The role of telomere dysfunction in genomic instability and age-related diseases
Genome Instability & Disease (2021)
-
Revesz syndrome revisited
Orphanet Journal of Rare Diseases (2020)